Signalment:
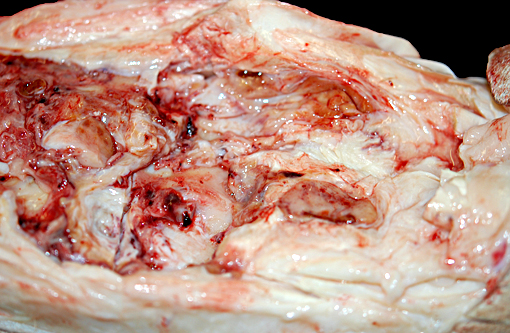
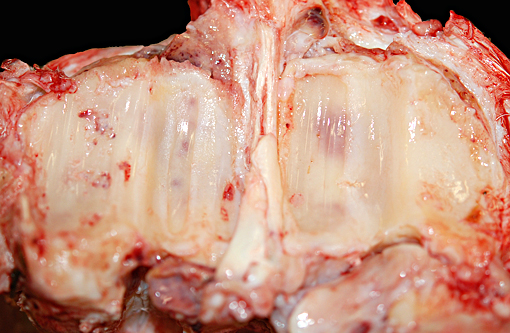
Gross Description:
Additional gross findings in this animal included mild to moderate, chronic active osteoarthritis in multiple joints with varying degrees of fibrinous effusion and synovial hyperplasia, multifocal renal cortical fibrosis, severe dental disease, and a benign ovarian tumor.
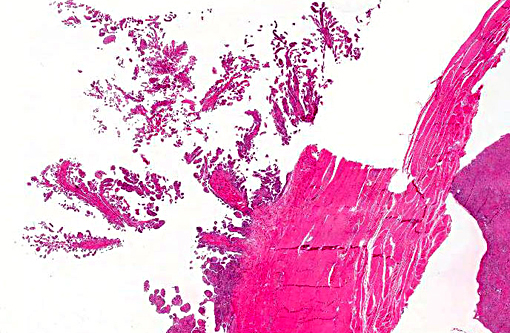
Histopathologic Description:
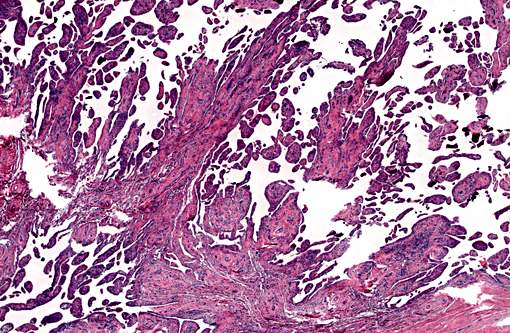
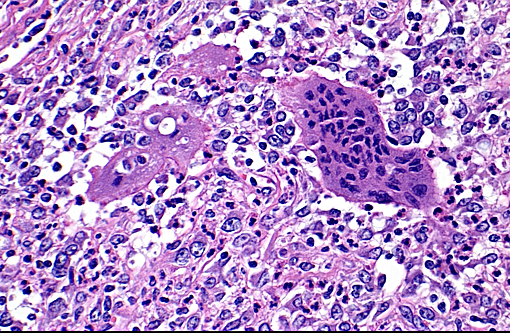
The synovium of the affected fetlock is diffusely and markedly hyperplastic with numerous thick frond-like villous projections. The synovium is expanded by a large population of mixed inflammatory cells with large numbers of neutrophils, and moderate granulation tissue. Occasionally, hyperplastic synovial villi contain large multinucleated cells, which are morphologically identical to those seen within the nodule in the adjacent tendon sheath.
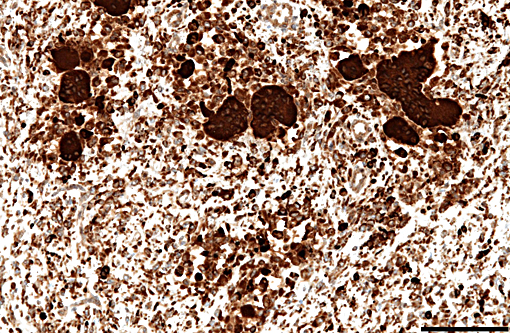
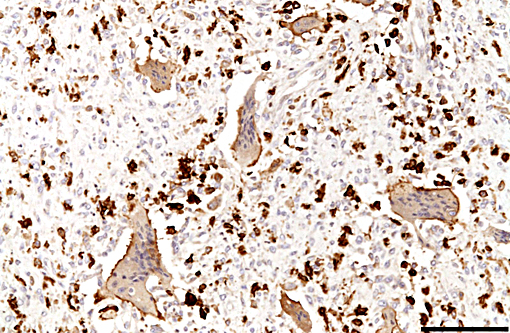
Immunohistochemically, large multinucleated cells all exhibit strong, cytoplasmic reactivity for cathepsin K and moderate cell membrane reactivity for IBA-1 (ionized calcium-binding adapter molecule 1). Multinucleated cells were negative for lysozyme immunostaining. Mononuclear, macrophage-like cells throughout the mass exhibit strong cytoplasmic reactivity for cathepsin K, while a smaller proportion of mononuclear cells showed moderate cyto-plasmic reactivity for IBA-1 and lysozyme.
Morphologic Diagnosis:
Condition:
Contributor Comment:
The etiology of this condition is uncertain, with debate over whether these lesions represent a primarily neoplastic or inflammatory process. Although these lesions are considered almost universally benign, rare reports of distant metastasis have occurred,(1) and localized forms may exhibit local recurrence after surgical excision.(8) Genetic analysis of human cases of PVNS has revealed a cluster of non-random clonal chromosomal translocations, with multiple cases sharing similar breakpoints on several chromosomes.(6) Interestingly, a majority of lesions share a break in the region of chromosome 1 which encodes macrophage colony stimulating factor (CSF1), a cytokine important for macrophage differentiation and proliferation. CSF1 is known to play an important role in the pathogenesis of numerous inflammatory arthritides, including rheumatoid arthritis. Recent theories on PVNS suggest a landscaping effect as part of the pathogenesis, with a low number of neoplastic cells overexpressing CSF1, resulting in recruitment of large numbers of mononuclear inflammatory cells which make up the bulk of the lesion.(4, 9)
In humans, 40-75% of PVNS cases have a history of previous trauma to the affected joint.(5) Similar to this giraffe, lesions in people occur most frequently in high use joints of the distal limbs, and are more frequently associated with the flexor surface. In this giraffe, no evidence of trauma was found grossly or radiographically at necropsy. However, based on the extended history, an episode of previous acute trauma or repetitive microtrauma cannot be excluded. Cultures of the synovial fluid from the affected joint were negative, and special stains of microscopic specimens were all negative for infectious organisms, making an infectious etiology unlikely.
The histogenesis of the large multinucleated cells remains elusive. Several cell types of origin have been proposed, including syno-vioblastic mesenchyme, synoviocytes, tissue macrophages, and cells of osteoclastic line-age. In the case of this animal, IHC yielded inconclusive results as to the origin of these unusual cells, with multinucleated cells expressing IBA1 (a cytoplasmic marker of macrophages) predominantly along the cell membrane, and showing diffuse strong cytoplasmic positivity for cathepsin K (a cysteine protease responsible for matrix-degradation). While cathepsin K has tradi-tionally been used as an osteoclast marker, recent reports have shown expression is not limited to osteoclasts. Cathepsin K expression has also been demonstrated in conditions resulting in high macrophage activation, such as granulomatous diseases (tuberculosis and foreign-body granulomas), sarcoidosis and sarcoid-like lesions, and is expressed by both macrophages and multinucleated giant cells in these conditions.(2) In the case of this giraffe, high expression of cathepsin K by cells in the tendon sheath and synovium may have contributed to osteoarthritic changes.
Inflammation and degenerative changes were also seen in other joints namely, the contralateral fetlock, both hocks, and the right stifle. Lesions were characterized by a range of changes, from multifocal cartilage fibrillation and erosion to focal chondromalacia, effusive synovitis/bursitis with fibrin and synovial villous hyperplasia. These lesions likely represent the effects of chronic forelimb lameness resulting in altered weight bearing in the other limbs as a compensatory response.
JPC Diagnosis:
Conference Comment:
Conference participants described the nodular mass as fibrovascular tissue containing numerous inflammatory cells including neutrophils, macrophages, many with hemosiderin, and multinucleate giant cells with fewer lymphocytes and plasma cells. The nodule also has multifocal areas containing fibrin, hemorrhage and edema. Fibroblasts or fibroblast like cells are hypertrophic and multifocally hyperplastic, and the collagenous stroma is haphazardly arranged and appears fibrillated in many areas; some participants described the histologic features of the nodular mass as pseudoneoplastic. Within sections of tendon histologic findings include degeneration and loss of nuclei, areas of hypercellularity, fraying of fibers, and side-to-side fusion and necrosis, all indicative of degeneration. The synovium is observed as proliferative and inflamed; the conference moderator discussed that because the synovium lines both tendons and joint spaces, that a reactive and inflamed synovium can result in the formation of adhesions. Conference participants speculated as to whether the villous proliferation of synovium originated from synovium lining a tendon, or from the synovium lining the joint space; ultimately participants concluded it was difficult to determine with certainty in this case.
This interesting case also was studied in consultation with the Department of Soft Tissue Pathology at the Joint Pathology Center, whose medical pathologists are familiar with tenosynovial giant cell tumor as described in humans. The medical pathologists agreed the histomorphologic features from the lesion in this giraffe resemble those of tenosynovial giant cell tumor as it occurs in humans; they also observed sheets of plump polygonal cells admixed with osteoclast type giant cells in a collagenized stroma. The medical pathologists also observed a neutrophilic and plasma cell infiltrate in the lesion, and commented that this is not a typical finding of tenosynovial giant cell tumor in people. They speculated that in the case of this giraffe, the inflammatory component may be indicative of an unrecognized infection, an autoimmune component, or possibly inflammatory recruitment by the tumor cells.
This is an interesting case in that the nature, origin and cause of the nodular lesion is unclear. As described above, there is indeed uncertainty in the literature regarding whether this is a neoplastic or inflammatory lesion. As seen in this case and described in others, the nodular proliferation is histologically distinct and different from the adjacent reactive hyperplastic villous synovium. When described as a neoplasm, many different patterns have been described in the literature, with the common thread being the presence of multinucleated giant cells.(7) The nodules have been described as proliferations of synovial cells, admixed with inflammatory cells including multinucleate giant cells,(3) with the cell of origin described as synoviocytes without anapestic features(7) or synovioblastic mesenchyme.(5) In other reports. the giant cells have been described as a proliferation of neoplastic epithelioid to pleomorphic mononuclear cells admixed with fibroblast like cells.(5) In this giraffe it is unclear if the nodules viewed histologically originated from the tendon sheath, the joint capsule, or from both locations; at necropsy, nodules were described grossly by the contributor as associated with both the tendon sheath and the joint capsule. Exact anatomic origin or location within or adjacent to the joint may not be critical, as it is recognized that there is an overlap in the appearance of lesions whether described as giant cell tumor of tendon sheath, localized nodular tenosynovitis, or pigmented villonodular synovitis of joints.(7)
References:
1. Asano N, Yoshida A, Kobayashi E, Yamaguchi T, Kawai A. Multiple metastases from histologically benign intraarticular diffuse type tenosynovial giant cell tumor: a case report. Hum Pathol. 2014;45: 23552358.
2. B+â-+hling F, et al. Cathepsin K--a marker of macrophage differentiation? J Pathol .2001;195: 375382.
3. Campbell MW, Koehler JW, Weiss RC, Christopherson PW. Cytologic findings from a benign giant cell tumor of the tendon sheath in a dog. Vet Clin Pathol. 2014;43: 2705.
4. Cupp J S et al. Translocation and expression of CSF1 in pigmented villonodular synovitis, tenosynovial giant cell tumor, rheumatoid arthritis and other reactive synovitides. Am J Surg Pathol. 2007;31: 970976.
5. Malatesta D. et al. Benign giant cell tumour of tendon sheaths in a European lynx (Lynx lynx). J Vet Med Ser A. 2005;52:125130.
6. Nilsson M. et al. Molecular cytogenetic mapping of recurrent chromosomal breakpoints in tenosynovial giant cell tumors. Virchows Arch. 2002;441:475480.
7. Pool RR, Thompson KG. Tumors of joints. In: Meuten DJ ed. Tumors of Domestic Animals. 4th ed. Ames, IA:Iowa State Press; 2002: 201-209.
8. Rao AS, Vigorita V J. Pigmented villonodular synovitis (giant-cell tumor of the tendon sheath and synovial membrane), A review of eighty-one cases. J. Bone Joint Surg. 1984;66:7694.
9. West R B, et al. A landscape effect in tenosynovial giant-cell tumor from activation of CSF1 expression by a translocation in a minority of tumor cells. Proc. Natl. Acad. Sci. 2006;103:690695.