Signalment:
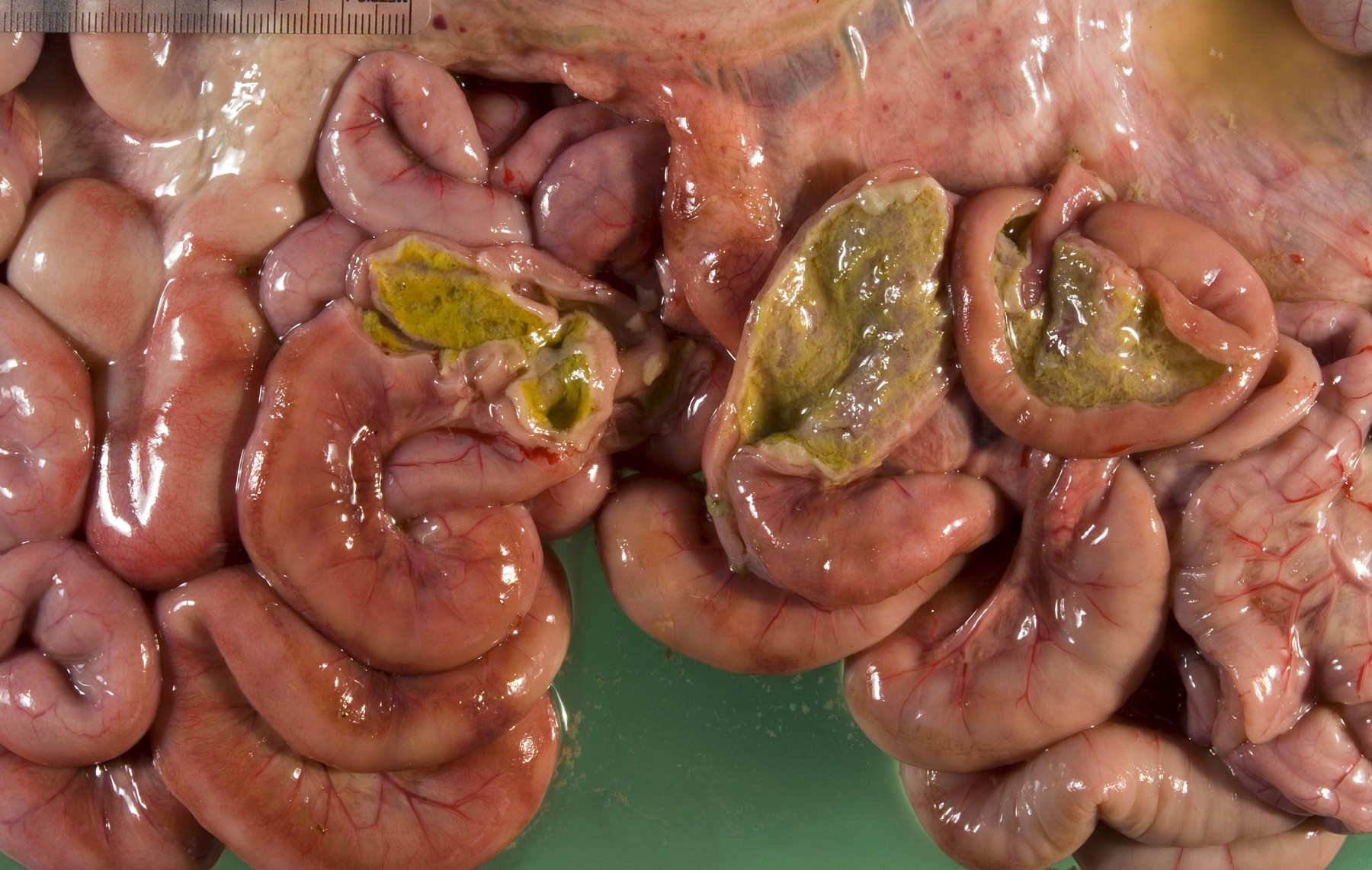
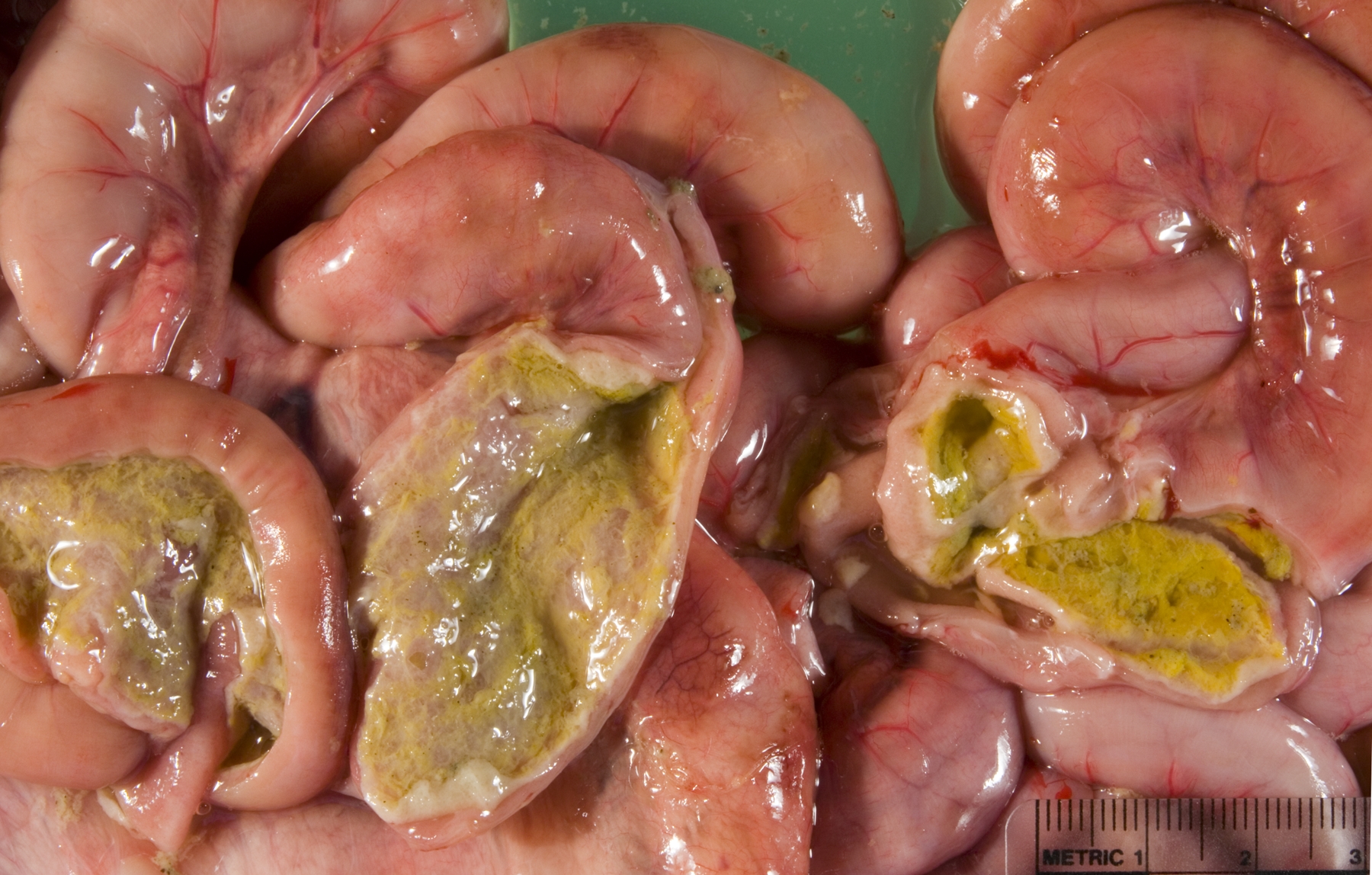
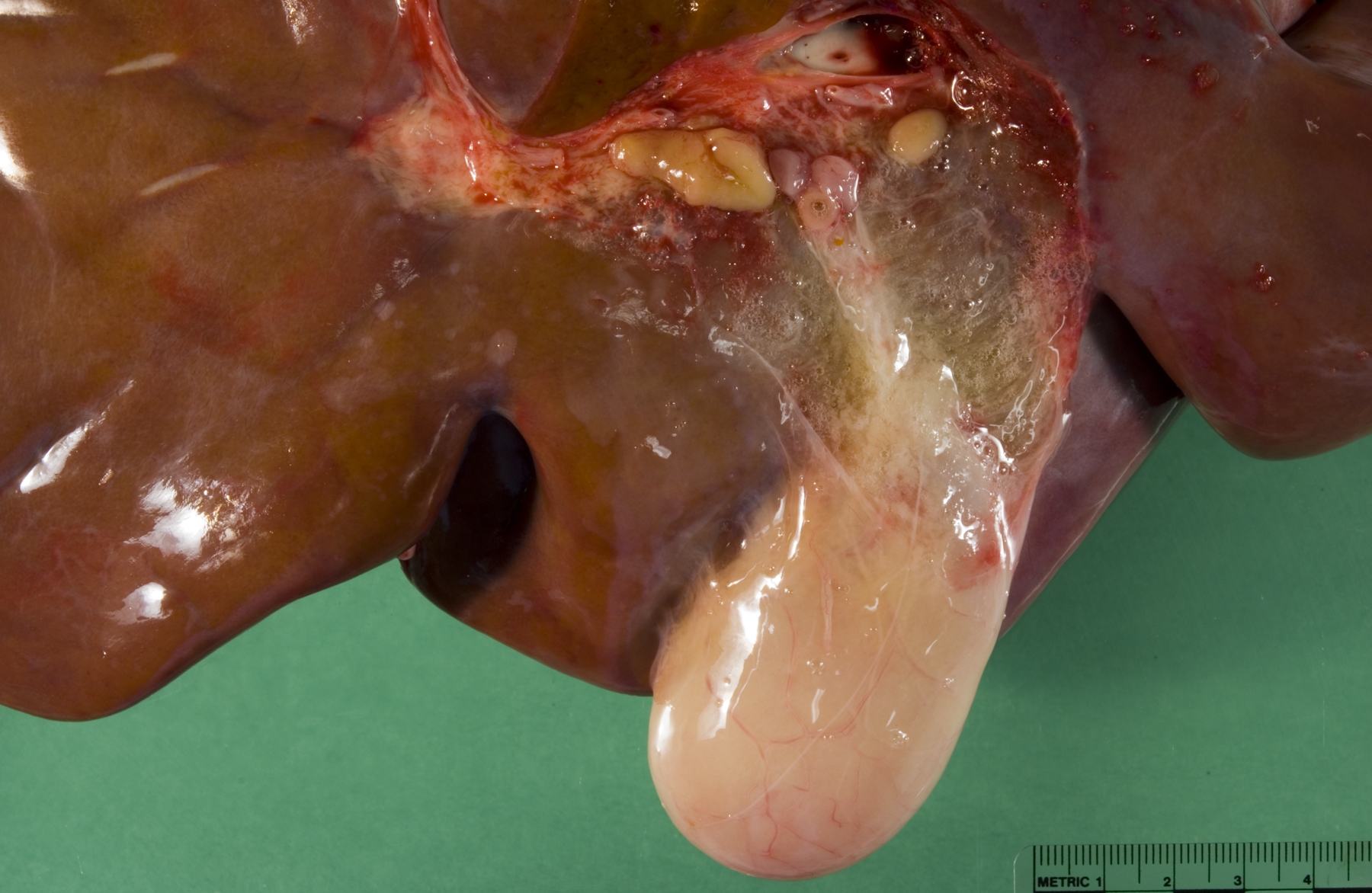
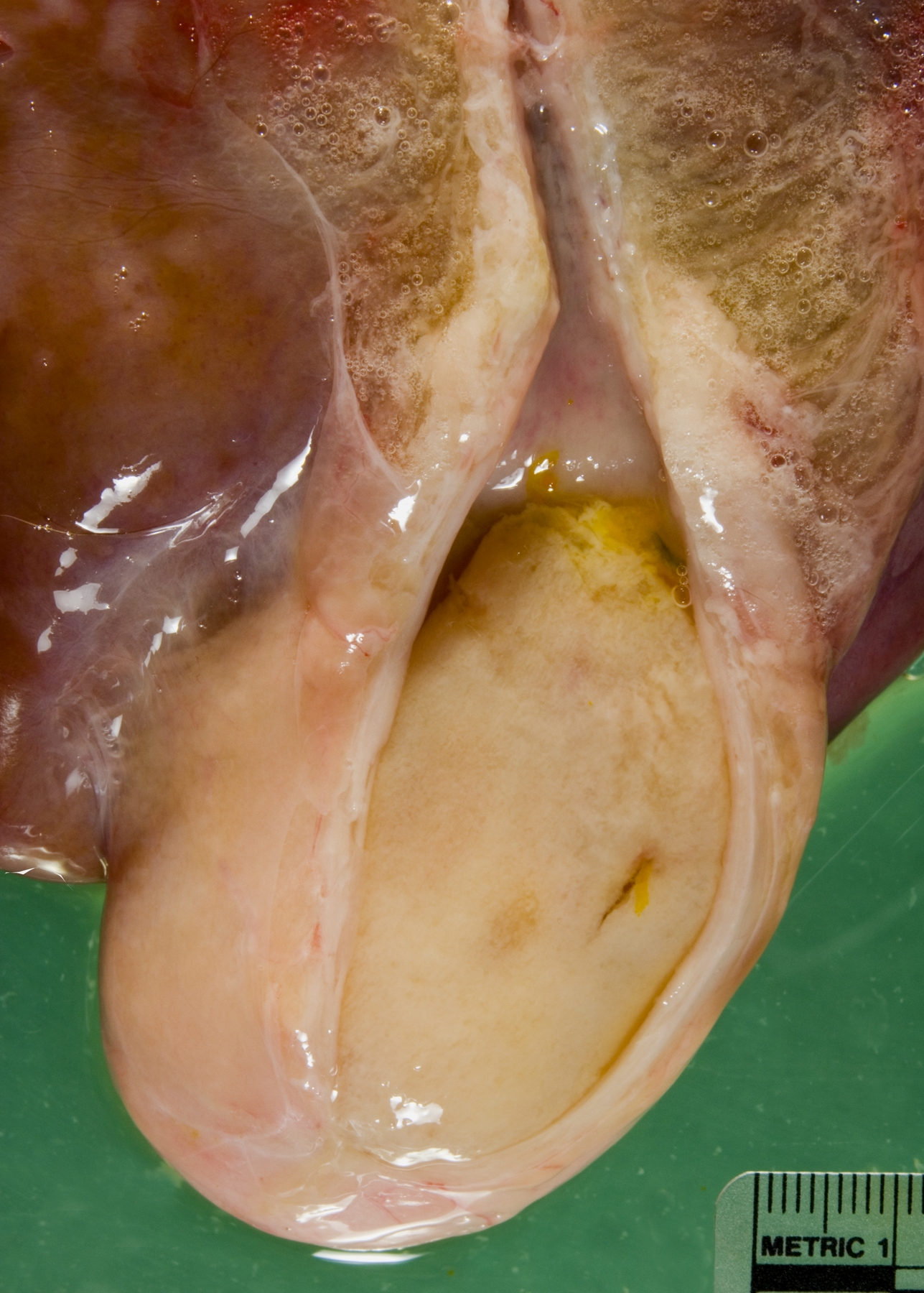
Gross Description:
Histopathologic Description:
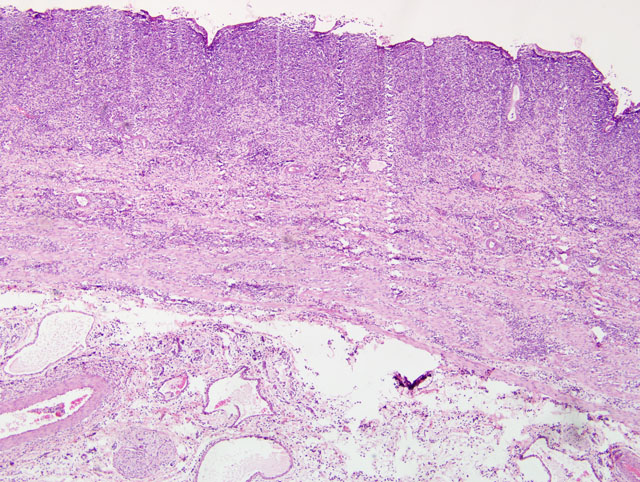
Gallbladder: The mucosa is expanded by infiltration of lymphocytes, macrophages, plasma cells and fewer neutrophils mixed with fibrin. Some capillaries and venules contain fibrin thrombi or are dilated and filled with neutrophils. Leukocytes also infiltrate the wall of some venules. The mucosal epithelium is multifocally attenuated or lost and has a few clusters of coccobacilli. A pleocellular (mostly lymphohistiocytic) inflammatory infiltrate dissects the tunica muscularis and infiltrates the edematous adventitia.
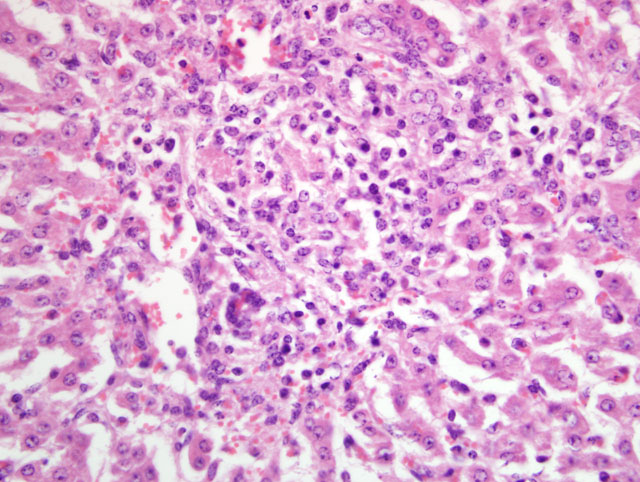
Liver: Periportal inflammation consists of lymphocytes and macrophages with rare neutrophils. This infiltrate is particularly intense around bile ductules, some of which have attenuated epithelium and lumina distended by bile. Random foci of lytic hepatocellular necrosis are infiltrated by histiocytes, lymphocytes, and few neutrophils. Vascular lesions are similar to those in the gallbladder. There are increased numbers of sinusoidal leukocytes and hypertrophy of Kupffer cells.
Small intestine: A thick layer of fibrin, neutrophils, degenerated leukocytes, and red blood cells with abundant Gram-negative bacterial coccobacilli and Gram-positive robust bacilli covers the focally denuded mucosa. Villi are blunted; goblet cells are scarce. The lamina propria is congested with numerous lymphocytes and histiocytes. Dilated crypts contain neutrophils and cellular debris. Lymphoid follicles of Peyers patches are depleted of lymphocytes with replacement by reticulohistiocytic cells. Vascular lesions are similar to those in the gallbladder.
Morphologic Diagnosis:
1. Small intestine: Fibrinosuppurative and necrotizing enteritis.
2. Gallbladder: Ulcerative and fibrinous cholecystitis.
3. Liver: Lymphohistiocytic cholangiohepatitis and necrotizing hepatitis.
Lab Results:
Condition:
Contributor Comment:
Calves with diarrhea and carrier cattle, including adult cattle, are the source for the fecal-oral route of infection.(2) Salmonella bacteria first colonize the distal small intestine, where pili facilitate attachment to the surface of enterocytes. The bacteria are taken up by enterocytes, macrophages and neutrophils. Production of enterotoxin results in the secretory diarrhea of enteric salmonellosis with loss of fluid and electrolytes; endotoxin production is important in septicemic salmonellosis. Some calves develop both syndromes (diarrhea and septicemia).(2)
The presence of gross lesions at necropsy examination helps to distinguish salmonellosis from enteric colibacillosis. Enteritis with yellow fibrinous exudate covering the mucosa is usually most severe in the ileum.(1) Mesenteric lymph nodes are enlarged and edematous.(1,2) The gallbladder is distended with an edematous wall and fibrinous mucosal inflammation.(2) Gray-white foci of hepatic necrosis may be observed macroscopically,(2) but were not obvious in this calf. These -�-�paratyphoid nodules are easier to detect histologically as foci of lytic necrosis with infiltration by a few macrophages.(1,2) Lymphoid depletion is obvious in the mesenteric nodes and intestine; fibrin thrombi in capillaries and venules with phlebitis may be observed in the intestine, liver, gallbladder or other tissues. (1,2)
JPC Diagnosis:
1. Small intestine: Enteritis, fibrinonecrotic, subacute, diffuse, severe, with erosions, diphtheritic membrane, and edema.
2. Gallbladder: Cholecystitis, transmural, fibrinonecrotic, diffuse, severe, with edema.
3. Liver: Cholangiohepatitis, lymphohistiocytic and neutrophilic, diffuse, mild to moderate, with multifocal, random lytic hepatic necrosis (paratyphoid nodules).
Conference Comment:
There are now two recognized species of Salmonella: S. enterica and S. bongori. S. enterica has six subspecies: enterica, salame, arizonae, diarizonae, indica and houtenae. S. enterica enterica causes a majority of the cases of salmonellosis in humans and domestic animals, and contains approximately 60% of the more than 2,200 distinct serovars and serotypes of Salmonella that have been identified. Besides S. Dublin, other important serovars include S. Enteritidis, S. Pullorum-Gallinarum, S. Arizonae., S. Choleraesuis, S. Typhisuis, S. Typhi, and S. Typhimurium (especially the multi-drug resistant definitive phage type 104 (DT104)). S. bongori are primarily recognized as tropical strains.(1)
An informative review by Santos et al(3) details the fascinating mechanisms by which the non-typhoidal Salmonella (NTS) serotypes adapt and thrive in the milieu of the inflamed intestine. Briefly, two key abilities enable S. Typhimurium to incite inflammation: 1) penetration of intestinal epithelium, and 2) survival within macrophages. These traits are conferred by two type III secretion systems (T3SS): T3SS-1, the invasion-associated T3SS encoded by Salmonella pathogenicity island (SPI) 1; and T3SS-2, responsible for survival in macrophages, encoded by SPI2. Mucosal barrier functions against NTS dissemination are activated via the interleukin (IL)-18/interferon-γ and IL-23/IL-17 axes, but the pathogen is adapted to survive the antimicrobial defenses of the inflamed intestininal lumen, allowing perpetuation of fecal/oral transmission.(3)
In general, three mechanisms account for the diarrhea. The first is malabsorption due to villous atrophy; there is reduced surface area for digestion and uptake of nutrients which then remain in the intestinal lumen exerting an osmotic effect which draws water. Second is secretory diarrhea due to an imbalance that results in a net excess of secretion over absorption; this is most often due to diarrheagenic enterotoxins of pathogenic bacteria, two classic examples of which are Vibrio cholera and Escherichia coli. The enterotoxins typically alter normal cellular secretory and absorptive functions of enterocytes. The final mechanism is effusive diarrhea, wherein there are changes in Starling forces or increased vascular permeability which allow fluid and solutes to move in a retrograde manner from the lateral intercellular space to the lumen. Interestingly, salmonellosis causes diarrhea via all three of these mechanisms. The secretory component is due to effector proteins, such as those encoded by SPI1 and SPI5, which block chloride channel closure or promote hypersecretion of chloride by enterocytes, respectively. Malabsorption is due to reduction of mucosal surface area and enterocyte function. Effusion of inflammatory exudates is the final component of this process.(1,2)
References:
2. Fenwick SG, Collett MG: Bovine salmonellosis. In: Infectious Diseases of Livestock, eds. Coetzer JAW, Tustin RC, 3rd ed., vol. 3, pp. 1582-1593. Oxford University Press, Cape Town, South Africa, 2004
3. Santos RL, Raffatellu M, Bevins CL, Adams LG, T+�-+kel +�-�, Tsolis R, B+�-�umler AJ: Life in the inflamed intestine, Salmonella style. Trends Microbiol 17:498-506, 2009