Wednesday Slide Conference, 2025-2026, Conference 3, Case 1
Signalment:
5.5-year-old, male castrated, domestic shorthair cat (Felis catus)History:
The cat was presented to the emergency service for a two-day history of lethargy and anorexia and a one-day history of labored breathing. The cat was reportedly indoor-only with no other animals in the house, had no history of illness, and had not been given any medications or preventatives. Thoracic auscultation revealed muffled heart sounds, decreased ventral lung sounds, and harsh dorsal lung sounds. The cat’s pulses were strong and synchronous, and no murmur or arrhythmias were appreciated. A brief thoracic ultrasound revealed a large amount of pleural effusion. Following a discussion about differentials and prognosis, the owner elected humane euthanasia with necropsy.Gross Pathology:
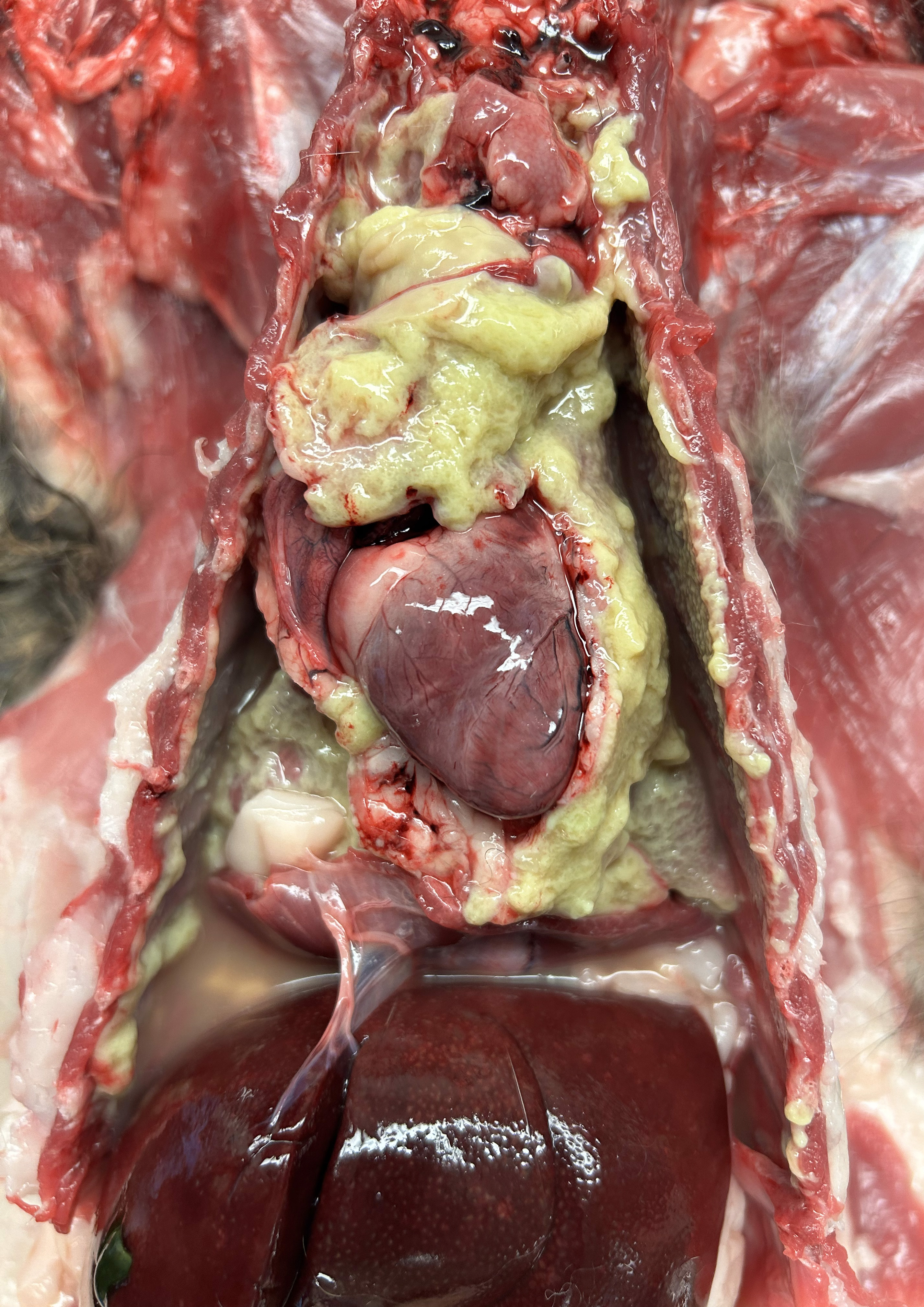
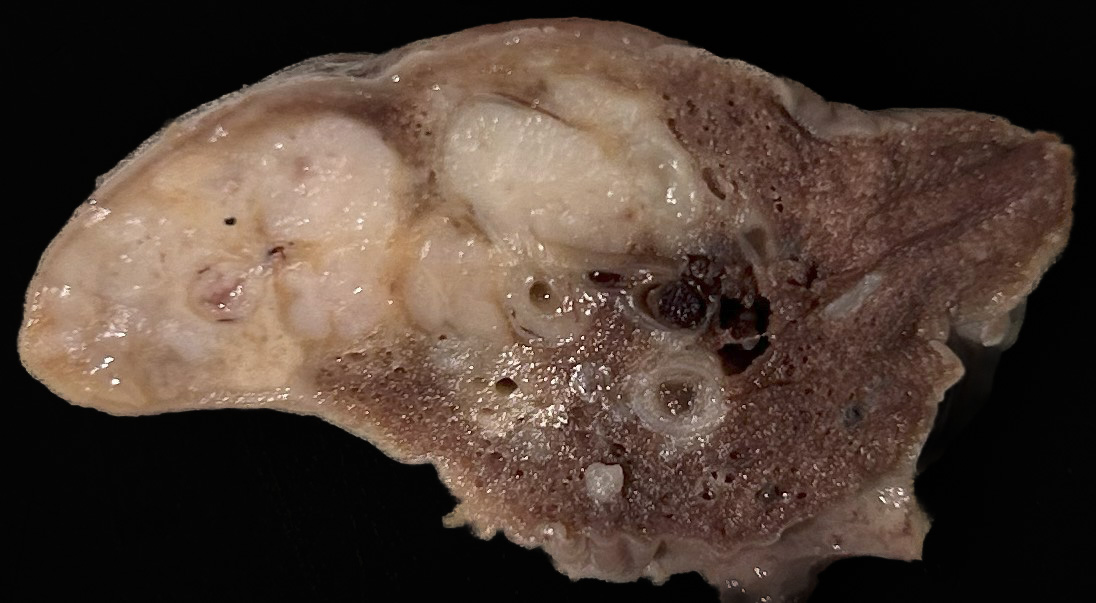
The thoracic cavity contained 180 mL of malodorous, tan to dark yellow to pale green, opaque effusion with free-floating aggregates of yellow to pale green friable material admixed. The same material covered the pericardium and multifocally covered the parietal and visceral pleura throughout the thoracic cavity, with the right side more severely affected than the left. The lung lobes were diffusely mildly rubbery and collapsed. The cranial aspect of the right caudal lung lobe was expanded by multiple, coalescing, approximately 0.3 cm diameter, tan to yellow nodules that oozed opaque, mucoid, yellow to gray exudate on cut section. Sections of the cranial portion of the right caudal lung lobe hovered below the surface in 10% formalin, while sections from the other lung lobes floated in 10% formalin.There was no evidence of thoracic trauma or migrating foreign material on gross postmortem examination.
Laboratory Results:
Aerobic culture of the pleural fluid isolated Escherichia coli, Actinomyces sp. (suspect Canibacter oris), and Staphylococcus aureus. Aerobic culture of fresh lung tissue isolated Escherichia coli. Anaerobic culture of both the pleural fluid and lung tissue had no growth.Microscopic Description:
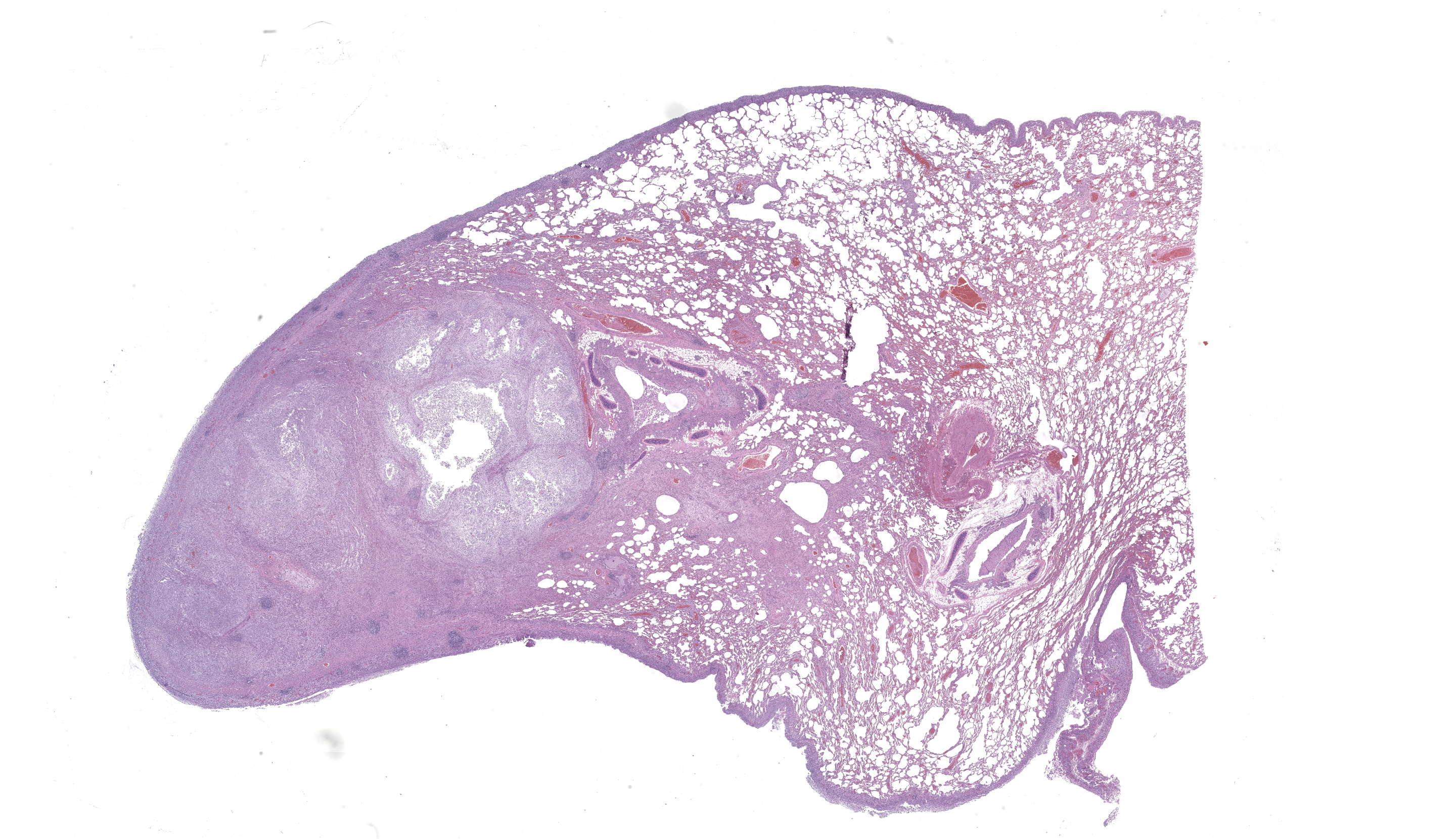
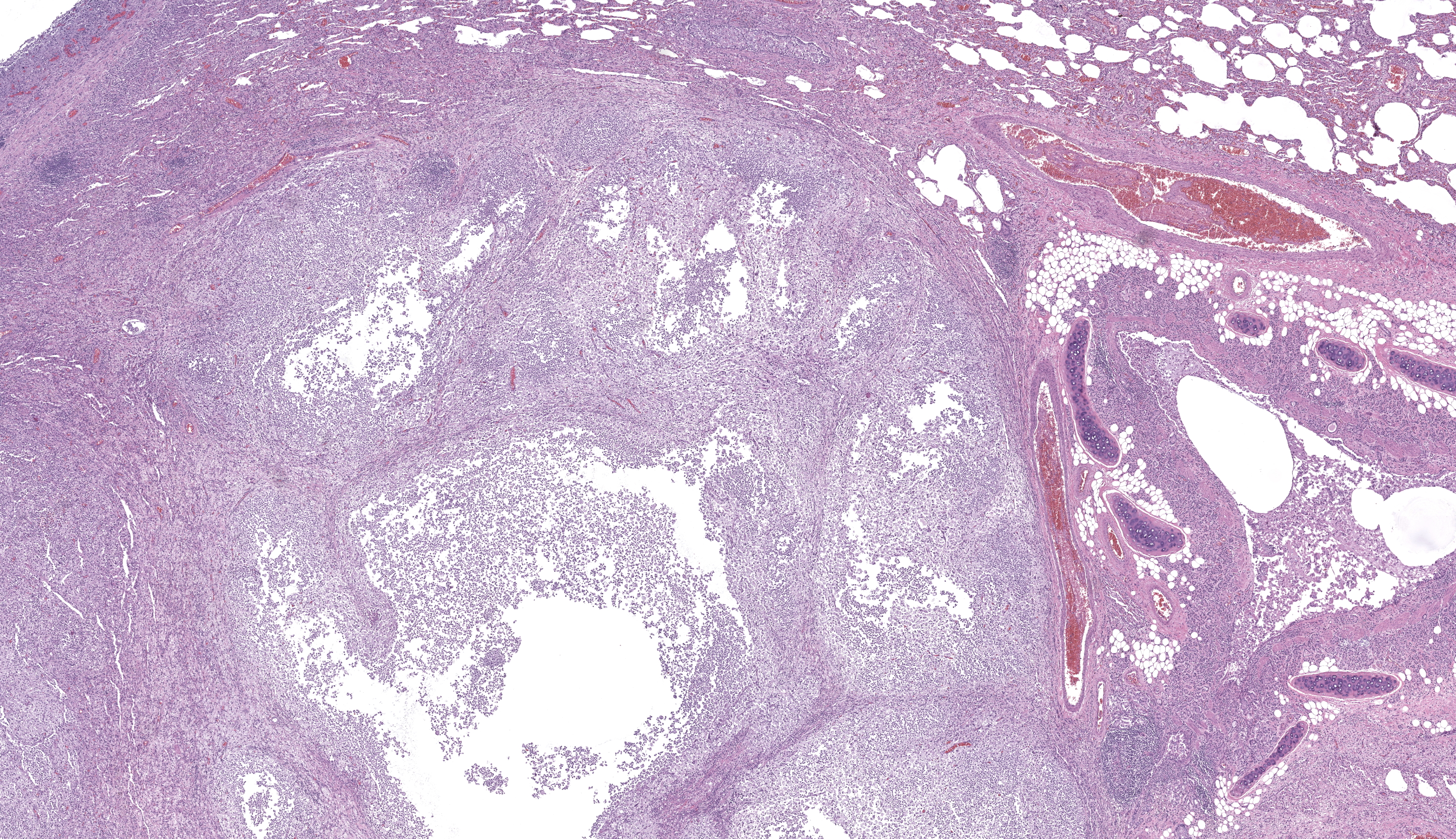
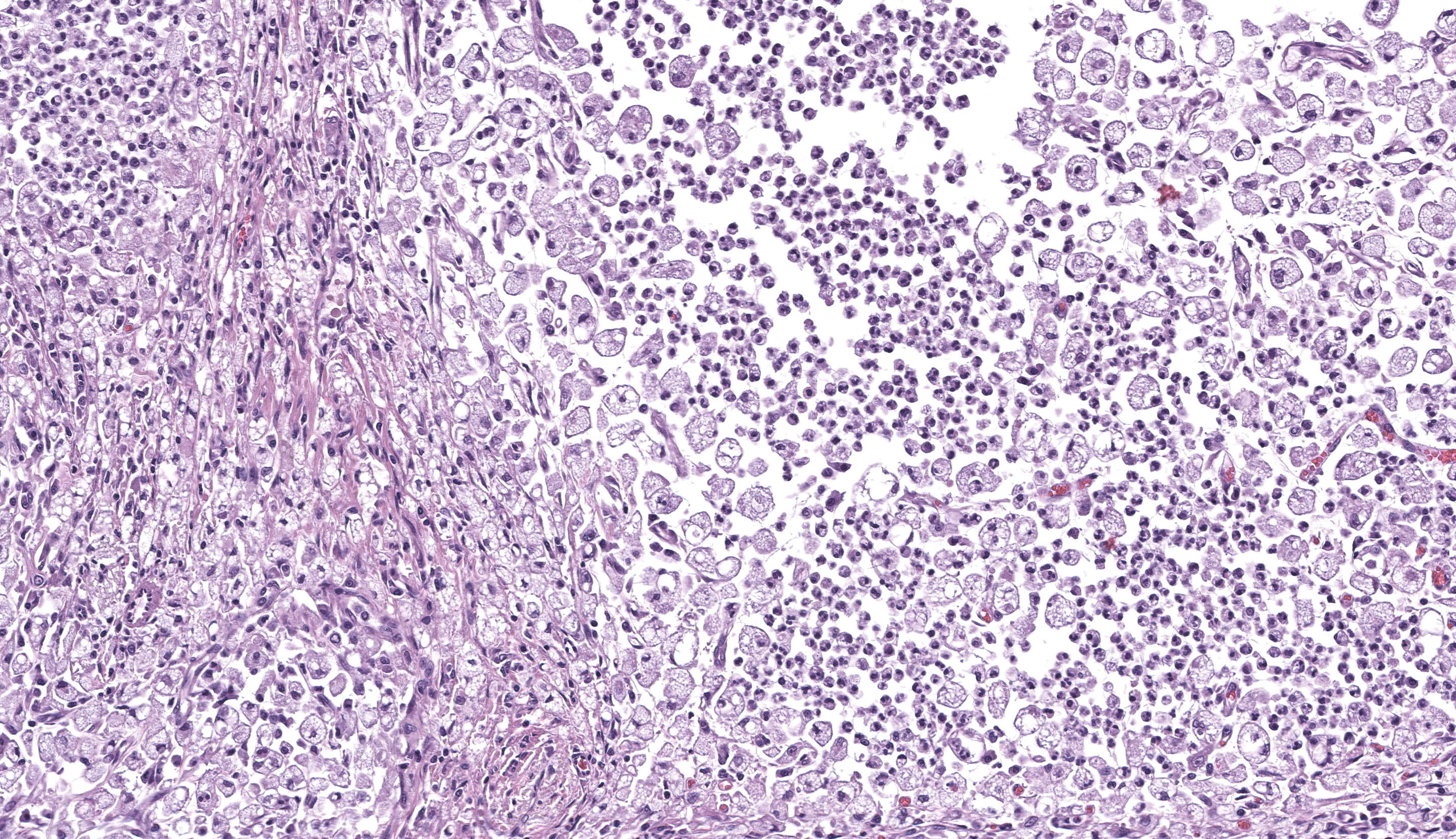
One section each of the right caudal lung lobe and pericardium are examined. The parenchyma of the right caudal lung lobe is severely expanded by coalescing aggregates of viable and nonviable neutrophils amidst abundant fibrin and cellular debris, admixed with fewer epithelioid macrophages and occasional multinucleated giant cells. These aggregates are further encircled by a ring of maturing fibrous connective tissue with few admixed lymphocytes and plasma cells. These foci of inflammation are centered on large airways and rarely contain fragments of bronchiolar walls that have been effaced. Less affected bronchi and bronchioles occasionally have eroded to ulcerated epithelium with intraluminal coagula of fibrin, neutrophils, foamy macrophages, erythrocytes, and proteinaceous fluid. The visceral pleura is covered by a mat of viable and nonviable neutrophils with fewer epithelioid macrophages enmeshed in abundant fibrin, cellular debris, and mixed bacteria comprising cocci and short to filamentous bacilli. The underlying pleural connective tissue is diffusely thickened by granulation tissue with infiltrating epithelioid macrophages, lymphocytes, and plasma cells. The pericardial pleura is similarly affected.Gram-stained sections of the right caudal lung lobe and pericardium demonstrate myriad gram-positive and gram-negative filamentous and short bacilli bacteria, both extracellular and within the cytoplasm of macrophages associated with the parietal pericardium, pulmonary visceral pleura, and pulmonary parenchymal lesions. An acid-fast-stained section (Ziehl-Neelsen) of the pericardium demonstrates that the filamentous and bacilli bacteria are uniformly acid-fast negative.
Contributor's Morphologic Diagnoses:
Cat, right caudal lung lobe: severe regionally extensive chronic fibrinosuppurative and pyogranulomatous bronchopneumonia and pleuritis with bronchiectasis, granulation tissue, fibrosis, and bacilli and filamentous bacteria.Cat, pericardium: severe diffuse chronic fibrinosuppurative and pyogranulomatous pericarditis with granulation tissue and bacilli and filamentous bacteria.
Contributor's Comment:
Pyothorax, or thoracic empyema, is characterized by accumulation of suppurative exudate within the pleural space.1 Cats between 4-6 years old are predominantly affected, and no sex or breed predispositions have been identified.2–4 More than 80% of feline pyothoraces are associated with a mix of obligate anaerobes (e.g., Clostridium, Fusobacterium, and Bacteroides sp.) and facultative aerobic bacteria (e.g., Pasteurella and Actinomyces sp.) which are reported constituents of the normal feline oropharyngeal flora, though non-oropharyngeal bacteria (including Staphylococcus aureus and Escherichia coli) have been isolated in a smaller percentage of cases.4-9 It follows that pyothorax has frequently been associated with bite trauma which, by direct penetration of the thorax or by local extension of a subcutaneous abscess, can inoculate the pleural space with oropharyngeal bacteria.2,3,10 Other reported routes of infection in cases of pyothorax include esophageal rupture, inhaled or migrating foreign bodies, larval migrans, therapeutic thoracic interventions (e.g., thoracocentesis or thoracic surgery), and spread of inflammation from a distant site.3 While chest bite trauma remains an important risk factor for pyothorax, current evidence suggests that aspiration of oropharyngeal bacteria, leading to colonization of the lower respiratory tract and subsequent parapneumonic spread, is a more likely source of pleural infection in cats.3,4,11 This mechanism of infection is also the most common mechanism of infection in human anaerobic pyothorax and equine pleuropneumonia.4,12,13In this case, the distribution of lung lesions, i.e. a focal parenchymal lesion in the right caudal lobe as well as pleural lesions throughout all lobes, in context with the absence of chest trauma, is most suggestive of parapneumonic spread from bronchopneumonia arising from aspirated oropharyngeal bacteria. The bacterial isolates from the lung and pleural effusion are consistent with the findings in the gram- and acid-fast-stained sections of tissue and correspond to organisms reported in the feline pyothorax literature.
Contributing Institution:
University of Pennsylvania School of Veterinary Medicine, Department of Pathobiology, https://www.vet.upenn.edu/
JPC Diagnoses:
Lung: Bronchopneumonia, necrotizing and pyogranulomatous, chronic-active, focally extensive, severe, with pleural granulation tissue, bronchiectasis, and mixed bacteria.JPC Comment:
COL (Retired) Jeremy Bearrs, former director of the Joint Pathology Center, spearheaded today’s conference with a case that ended up as a staff favorite! This case’s discussion started off with some clarifying definitions of pneumonia-related terms that are used ubiquitously by the pathologist that have specific meanings and should be used accurately. These included: “bronchopneumonia”, referring to an exudative pulmonary lesion originating at the bronchiolar-alveolar junction; bronchopneumonias usually start via airborne entry to the respiratory tract, are most often seen in the cranioventral lung lobes grossly, and often have an inflammatory exudate filling airways; “interstitial pneumonia”, referring to generally diffuse destruction and inflammation centered upon the three components of the alveolar wall, frequently undergoing exudative, proliferative, and fibrosing phases, and typically enters the lung via bloodborne route of entry (think “viral”) or secondary to toxins; “bronchointerstitial pneumonia”, defined as the destruction of both bronchiolar and alveolar epithelium, typically via an airborne route of entry; “bronchitis”, defined as a type of pulmonary inflammation that affects the bronchi specifically (note that “bronchopneumonia” does NOT, by definition, include the bronchi); and “bronchiectasis/bronchiolectasis”, meaning a bronchus or bronchiole, respectively, is irreversibly expanded by inflammation and/or inspissated material as a sequela to loss of elastin and increased fibrosis from a chronic inflammatory response. These terms laid the groundwork for more descriptive accuracy and conversation by the conference participants. Among other notable points of discussion was a challenge to participants to name the major large colony-forming bacteria, which was prompted by the presence of sizeable bacterial colonies in the case slide. These bacteria include Yersinia, Actinomyces, Actinobacillus, Campylobacter, Corynebacterium, Staphylococcus, Streptococcus, Nocardia, and Trueperella (YAACCSS-NT). On a final note, Dr. Bearrs made an important point to mention knowing which bacteria are exceptions to general rules, such as Clostridium piliforme, which is one of very few Clostridial species that are gram-negative.Feline infectious peritonitis (FIP), congestive heart failure (CHF), pyothorax, and neoplasia represent the overwhelming majority of cases of pleural effusion in cats.4 In an elegantly succinct manner, the contributor wrote a terrific comment about routine causes of pyothorax in cats. The possibility of aspiration from a regurgitation that the owners may not have been aware of (because, you know, cats) is certainly worth considering given the presence of E. coli. While the pathogenesis of an aspiration pneumonia with pleural spread might be considered reasonably straightforward, understanding how any form of pulmonary inoculation, including aspiration, leads to pneumonia and parapneumonic disease is worth reviewing.
Normal bacterial flora can be symbiotic with their host when they stay where they’re supposed to be, but can become dangerous pathogens when they go where they aren’t. Considering the bacteria cultured from this case (E. coli, S. aureus, and Actinomyces spp), E. coli will be used as an example. There are a variety of non-pathogenic E. coli strains that reside in the GI tract of cats (and most mammals) that are generally considered beneficial. However, they can also be opportunistic when they end up in, say, the lungs because some kid thought it was funny to make their cat gag at the sound of tape being ripped and it threw up its breakfast. Any event of vomiting or regurgitation can result in an aspiration, though this is most commonly seen during sedation or anesthesia. Those beneficial gut bacteria, having been forcibly ejected from their home and sucked into the respiratory tract along with other bacteria from the oropharynx, find themselves in a strange and foreign place full of opportunity, far beyond the normal protective mechanisms of the coughing reflex and the mucociliary apparatus. Thus, the party begins. The presence of bacteria in the airways causes inflammation and it’s not long before the “cops” show up to try and handle the wayward bacteria. Alveolar macrophages, being the resident neighborhood watch, show up first, having been summoned to the scene by pro-inflammatory cytokines such as IL-1, IL-6, IL-8, and TNFα. However, these cells can only do much, so they start calling in additional support in the form of neutrophils. With all these inflammatory cells and their myeloperoxidases showing up, there is rapid accumulation of pus full of bacteria, dead cells, fibrin, fluid, and damaged tissue. That’s the short version of how a suppurative aspiration pneumonia is born.
As the bacteria continue to grow and translocate throughout the lungs, they eventually reach the pleural surface. From there, it’s just a hop, skip, and a jump into the pleural cavity. This is usually a potential space with just a minute amount of fluid within it to allow for lubrication during respiration, but the inflammation from the raging pneumonia can result in additional fluid accumulation and edema around the lungs.4 Bacteria can move out into that fluid and, where they go, neutrophils will follow and do what they do…kill things and make pus. In cats (and dogs), the mediastinum is fenestrated, so there is frequently bilateral accumulation of fluid.4 That suppurative pneumonia has now graduated to a pyothorax.
Pyothoraces can be notoriously challenging to treat and most require aggressive medical management +/- surgery. In cats, survival rates from pyothorax are reported to be only 62%, with a 58% complication rate with thoracotomy tube placement.11 Post-treatment complications are common and include pleural adhesions, pulmonary fibrosis, abscesses, sepsis, cardiac disease, recurrent infections, bronchopleural fistulas, and even death. However, parapneumonic disease in indoor cats from single-cat households is rare. The moral of the story? Keep your “murder mittens” in the house and, aside from the good that it would do for songbird populations, reduce their risk of pyothorax!
References:
- Barrs VR, Allan GS, Martin P, Beatty JA, Malik R. Feline pyothorax: a retrospective study of 27 cases in Australia. J Feline Med Surg. 2005;7:211–222.
- Barrs VR, Beatty JA. Feline pyothorax - new insights into an old problem: part 1. Aetiopathogenesis and diagnostic investigation. Vet J Lond Engl. 2009;179:163–170.
- Bartlett JG. Anaerobic Bacterial Infections of the Lung and Pleural Space. Clin Infect Dis. 1993;16:S248–S255.
- Beatty J, Barrs V. Pleural effusion in the cat: a practical approach to determining aetiology. J Feline Med Surg. 2010;12(9):693-707.
- Heier E, Wurtinger G, Hassdenteufel E, Schneider M. Therapy of Pyothorax in Cats via Small-Bore Thoracostomy Tube in Terms of Efficacy, Complications and Outcomes. Animals. 2022;12:107.
- Johnson LR, Epstein SE, Reagan KL. Etiology and effusion characteristics in 29 cats and 60 dogs with pyothorax (2010?2020). J Vet Intern Med. 2023;37:1155–1165.
- Lopez A, Martinson SA. Chapter 9: Respiratory System, Thoracic Cavities, Mediastinum, and Pleurae. In: Zachary, JF, ed. Pathologic basis of veterinary disease. 7th ed. Elsevier; 2022.
- Love DN, Jones RF, Bailey M, Johnson RS, Gamble N. Isolation and characterization of bacteria from pyothorax (empyema) in cats. Vet Microbiol. 1982;7:455–461.
- Love DN, Vekselstein R, Collings S. The obligate and facultatively anaerobic bacterial flora of the normal feline gingival margin. Vet Microbiol. 1990;22:267–275.
- Racklyeft D, Raidal S, Love D. Towards an understanding of equine pleuropneumonia: factors relevant for control. Aust Vet J. 2000;78:334–338.
- Sim JJ, Lau SF, Omar S, Watanabe M, Aslam MW. A Retrospective Study on Bacteriology, Clinicopathologic and Radiographic Features in 28 Cats Diagnosed with Pyothorax. Animals. 2021;11: 2286.
- Stillion JR, Letendre J. A clinical review of the pathophysiology, diagnosis, and treatment of pyothorax in dogs and cats. J Vet Emerg Crit Care. 2015;25:113–129.
- Waddell LS, Brady CA, Drobatz KJ. Risk factors, prognostic indicators, and outcome of pyothorax in cats: 80 cases (1986-1999). J Am Vet Med Assoc. 2002;221:819–824.
- Walker AL, Jang SS, Hirsh DC. Bacteria associated with pyothorax of dogs and cats: 98 cases (1989-1998). J Am Vet Med Assoc. 2000;216:359–363.