WSC 23-24 Conference 8, Case 2
Signalment:
6-month-old, female Yorkshire pig (Sus domesticus)
History:
Seven 6-month-old pigs were shipped to a research facility and all animals developed a high-grade fever and high respiratory rate one week after arrival. The animals were treated with antibiotics and nonsteroidal anti-inflammatories to reduce the fever. One died overnight and was submitted for necropsy.
Gross Pathology:
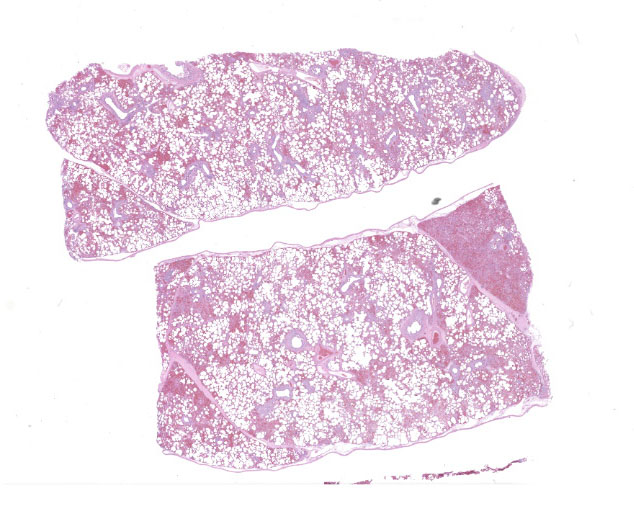
The lungs were diffusely mottled red to dark red, with patchy areas of consolidation on the right side.
Laboratory Results:
PCR positive for Porcine Reproductive and Respiratory Syndrome Virus.
PCR negative for Porcine circovirus-2, PCV-3, and Influenza A.
Lung culture resulted in moderate amounts of mixed bacterial growth.
Microscopic Description:
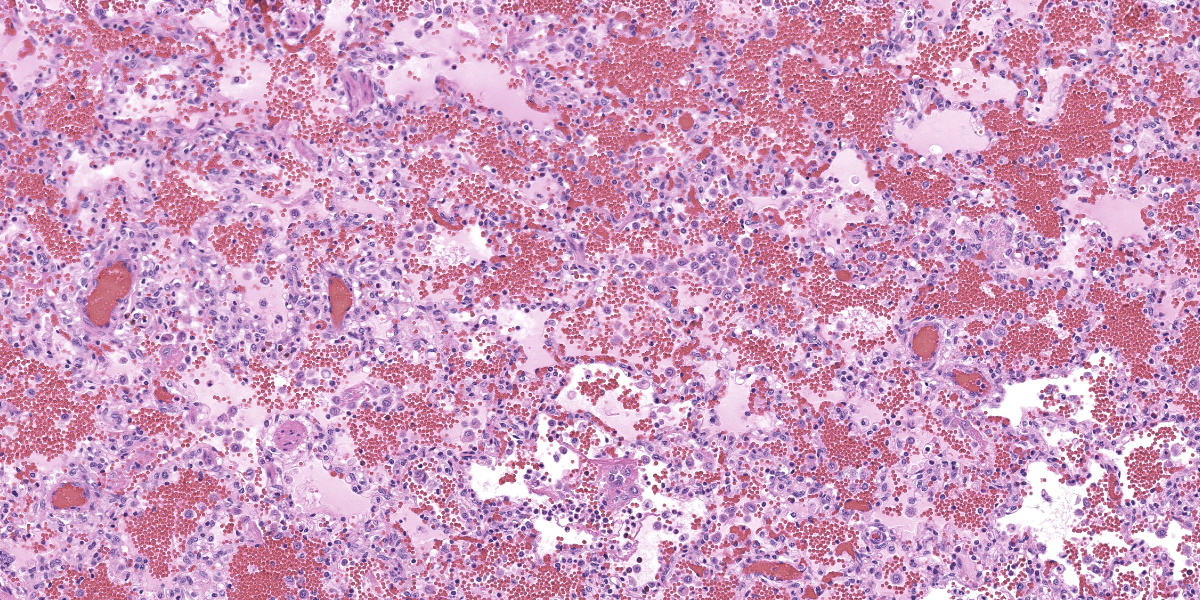
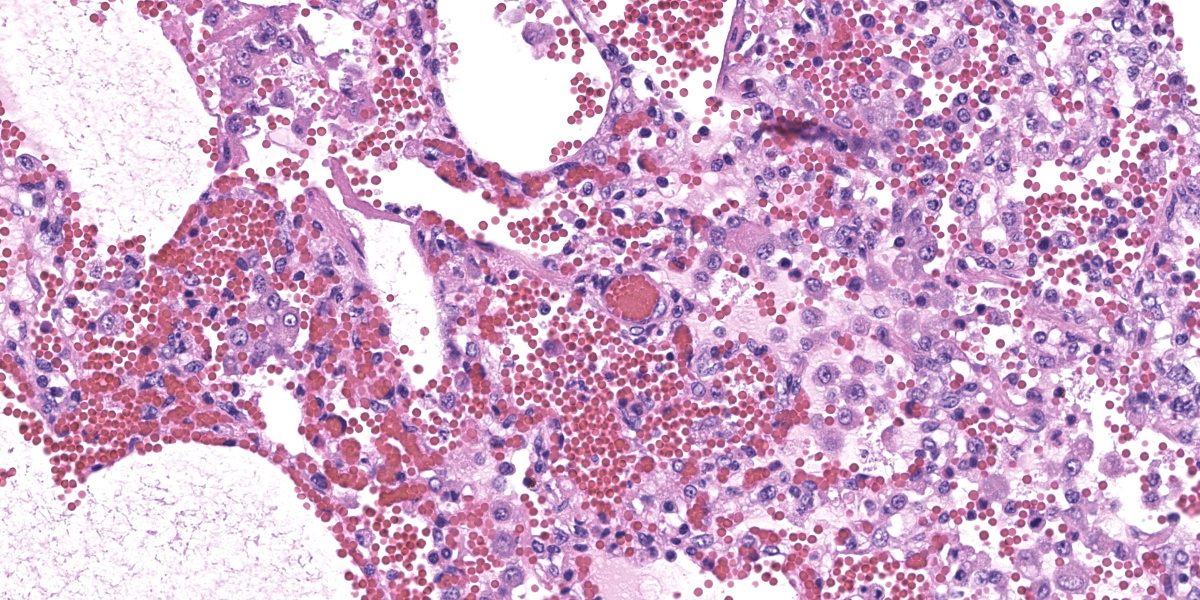
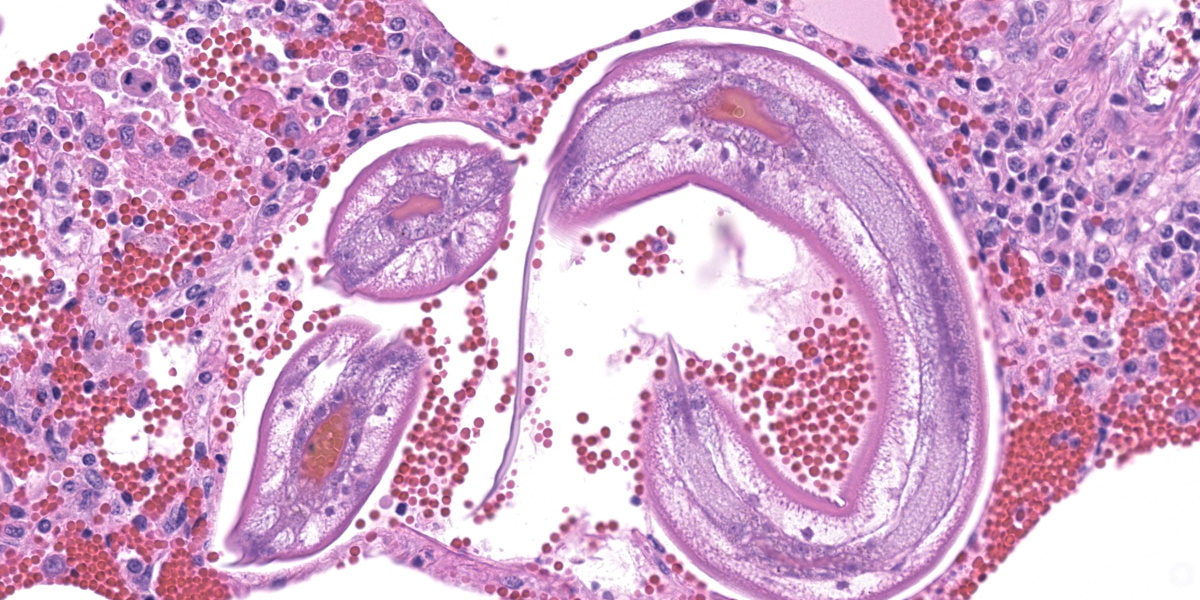
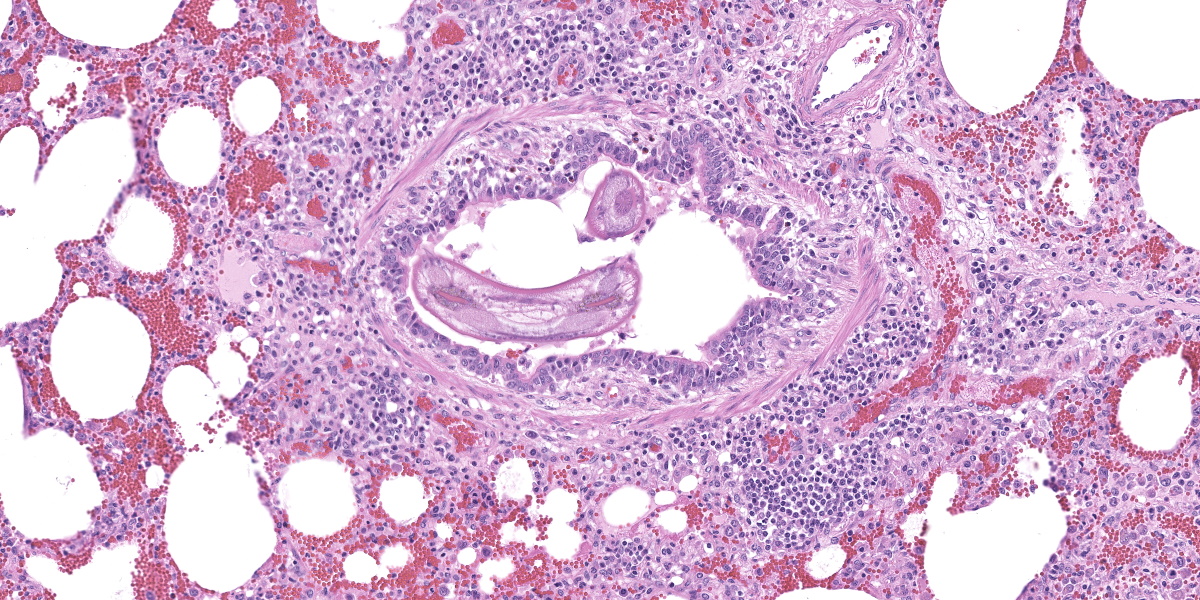
In multifocal to coalescent foci affecting approximately 30% of the examined area of lung, alveolar spaces are filled with erythrocytes, fibrin, large numbers of macrophages, fewer multinucleated giant cells, and scattered eosinophils and neutrophils. Adjacent alveolar septa are moderately to markedly widened by fibrin and similar inflammatory infiltrates, and occasionally form confluent ruptured spaces (emphysema). Multifocally, alveolar macrophages are hypereosinophilic and contain pyknotic nuclei (necrosis). The inflammatory infiltrate often extends into and fills terminal bronchioles, with expansion and disruption of the epithelium by inflammation. Affected bronchioles are lined incompletely by attenuated epithelium. Multifocally throughout affected and unaffected regions of lung are numerous nematode larvae within both alveoli and bronchioles. Nematode larvae are 40-60 µm in diameter, with a 3 µm thick smooth cuticle, prominent lateral alae, large lateral chords, and coelomyarian-polymyarian musculature. The pseudocoelom contains a polycytous intestine lined by uninucleate epithelial cells. Some bronchioles that contain nematodes are lined by variably hyperplastic to eroded epithelium infiltrated by increased numbers of eosinophils. Interlobular septa are expanded by edema.
Contributor’s Morphologic Diagnosis:
Lung: Interstitial pneumonia, histiocytic and eosinophilic, acute, multifocal to coalescing, moderate, with intra-alveolar macrophage necrosis, and intra-alveolar and intra-bronchiolar ascarid larvae consistent with Ascaris suum.
Contributor’s Comment:
While the patchy inflammatory infiltrate is occasionally centered on nematode larva in this case, interstitial pneumonia in pigs has several viral and bacterial differential diagnoses. The presence of necrotic alveolar macrophages increased the suspicion for Porcine Reproductive and Respiratory Syndrome (PRRS) virus, and the virus was detected in the lung by PCR.
PRRS virus is a porcine arterivirus with a wide variety of clinical manifestations in pig herds depending on the endemic nature of the virus in the herd, age, immune status, and presence of coinfecting pathogens.2 Most notably, the virus causes reproductive and respiratory disease. The virus infects macrophages at the point of entry and disseminates to local lymphoid tissue, resulting in viremia and systemic infection.7 Pulmonary lesions are characterized by interstitial pneumonia with expansion of the alveolar septa by increased numbers of mononuclear leukocytes.
Necrotic macrophages and smudged chromatin within alveoli are consistent findings in PRRS infection.2
PRRS virus infection has been reported to have immunosuppressive effects that enable secondary viral and bacterial infections.7 Multiple mechanisms of immunosuppression have been described, including reduction in NK cell cytotoxic activity, promotion of immunosuppressive cytokines IL-10 and TGF-B, and impairment of pulmonary macrophage functional activity.2,5,7Streptococcus suis, Glaesserella parasuis, and Salmonella spp. are common co-infecting pathogens, but an association with pulmonary parasitism or parasite migration has not been described.2
The intrapulmonary helminths in this case are morphologically consistent with ascarid nematodes. In combination with the signalment, the histologic features of the ascarid nematode (diameter, presence of prominent lateral alae, and appearance of the hypodermis, musculature, and intestinal tract) are compatible with larval Ascaris suum.4
Ascaris suum is ubiquitous in swine populations and infection results in production losses and potentially severe pathologic consequences.1 The life cycle, as with most ascarids, involves ingestion of the ascarid egg which hatches in the gastrointestinal tract. The larvae gain access to the portal vasculature, leading to larval migration through the hepatic parenchyma. From there, larvae access the pulmonary vasculature and enter alveoli. Eventually larvae ascend the bronchiolar tree and are coughed up and swallowed back into the gastrointestinal tract where they develop into sexually mature adults. Mechanical damage by the migrating larvae in the lung results in hemorrhage, edema, and eosinophil infiltration. As the larvae mature, an eosinophilic bronchiolitis ensues, with reactive hyperplasia or denuding of bronchiolar epithelium and invasion of eosinophils into bronchiolar walls.1,2
While an immunologic association between the PRRS infection and the ascarid infection could not be determined, this case highlights the importance of ruling out primary viral causes of pneumonia in pigs with pulmonary ascariasis.
Contributing Institution:
Washington State University
Department of Veterinary Microbiology and Pathology and the Washington Animal Disease Diagnostic Laboratory
https://waddl.vetmed.wsu.edu/ and https://vmp.vetmed.wsu.edu/
JPC Diagnoses:
- Lung: Pneumonia, interstitial, lymphohistiocytic, multifocal, marked, with type II pneumocyte hyperplasia and peribronchiolar and perivascular lymphoid hyperplasia.
- Lung: Pneumonia, interstitial, histiocytic and eosinophilic, multifocal, mild, with eosinophilic bronchiolitis and ascarid larvae.
JPC Comment:
Ascarids are nematodes of extremes. They are among the largest of the worms found in domestic animals, with members of the order Ascaridida ranging from several inches up to 2 feet in length.1 Ascarid eggs are also remarkably resistant to chemical and physical insults in the environment and can remain infective in soil for years.1
The life cycle of the terrestrial ascarids tend to be direct, and larvae undergo two molts within their hardy eggshell, emerging within the host as infective L3 larvae.1 Ascarids also tend to be relatively host specific, and most domestic species have at least one ascarid to call their very own; Parascaris equorum infects horses, Toxocara vitulorum infects cattle, Taxocara canis infects dogs, Toxocara cati infects cats, and, of course, Ascaris suum infects swine.1
There are exceptions to this “one ascarid, one host” rule. Historically, Ascaris suum was thought to be identical to Ascaris lumbricoides in humans, though these two organisms are now considered distinct species. Nevertheless, A. lumbricoides can mature in swine and A. suum can mature in humans, as evidenced by one recent study where 50 A. suum eggs were fed to healthy, human research subjects.1,3 Once they emerged from the required regulatory paperwork, researchers found that experimental infection produced clinical symptoms identical to A. lumbricoides infection, including respiratory discomfort and radiographic evidence of pulmonary larval migration, and A. suum eggs were recovered from the feces of the intrepid volunteers.3A. suum may also infect sheep, causing mild respiratory disturbances in young lambs. Calves exposed to yards contaminated with infective pig feces may develop severe acute interstitial pneumonia with millions of A. suum larvae present in the lungs.6
The contributor provides an excellent summary of the winding walkabout taken by the typical A. suum larva after emerging from its egg in the small intestine of its porcine host. In young pigs, extensive pulmonary damage can lead to severe respiratory disease, with rapid, shallow breaths and audible expiratory efforts (“thumps”) that may lead to death.1 As larvae wander through the liver, the mechanical damage and ensuing inflammation heal by fibrosis, producing “milk spots” that cause condemnation of the liver at slaughter.1 While less pathogenic, infection by adult worms may cause diarrhea, impaired nutritional uptake and growth, and rare disorders such as perforated bowels or bile duct occlusion.1 Taken together, these effects make
Ascaris suum the most economically important nematode of swine.
Dr. Gardiner reminded conference participants that nematode larvae are typically coiled; thus, when assessing parasite load on histology, it is helpful to remember that a close grouping of cross sections typically represents only one larva, not multiple. Dr. Gardiner also noted that conference participants failed to describe the nematode excretory cells, visible as eosinophilic material within the larval lateral cords.
Dr. Williams noted that you rarely find only one process in pig lungs; they tend to reward careful evaluation with additional revelations. In this case, while the nematode larvae may initially hog the spotlight, careful examination reveals multiple areas within the lungs that are devoid of nematodes but rich in macrophages, fibrin, hemorrhage, edema, and multinucleated cells. These lesions are not entirely attributable to nematode migration, and a young pig with many macrophages and multinucleated cells in the lungs should raise suspicion for PRRSV and PCV-2. Differentiating the two can be difficult, though large numbers of necrotic macrophages, while not apparent in the section examined at conference, are characteristic of PRRSV infection.
There was spirited discussion about whether to combine the nematode and PRRSV lesions into one morphologic diagnosis, particularly since assigning histologic effects to one etiology or another is somewhat artificial in this case. The separatists carried the day, however, as the majority of participants felt the etiologic agents were unrelated, each notable, and each deserving of their due.
References:
- Bowman DD. Georgis' Parasitology for Veterinarians. 10th ed. Elsevier;2014: 191-192.
- Caswell JL, Williams KJ. The respiratory system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 6th ed. Vol 2. Elsevier Saunders;2016:523-526.
- da Silva TE, Barbosa FS, Magalhaes LMD, et al. Unraveling Ascaris suum experimental infection in humans. Microbes Infect. 2021;23(8):104836.
- Gardiner CH, Poynton SL. An Atlas of Metazoan Parasites in Animal Tissues. Armed Forces Institute of Pathology/ American Registry of Pathology; 2006:2-29.
- Toman M, Celer V, Kavanova L, et al. Dynamics and differences in systemic and local immune responses after vaccination with inactivated and live commercial vaccines and subsequent subclinical infection with PRRS virus. Front Immunol 2019;10:1689.
- Uzal FA, Plattner BL, Hostetter JM. Alimentary System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 6th ed. Vol 2. Elsevier Saunders;2016:218-219.
- Zimmerman JJ, Benfield DA, Dee SA, et al. Porcine reproductive and respiratory syndrome (porcine arterivirus). In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, eds. Diseases of Swine. 10th ed. Blackwell Publishing;2012:52,461-480.