Wednesday Slide Conference, Conference 9, Case 2
Signalment:
13 year old, castrated male, Yorkshire terrier, Canis familiaris, Dog
History:
History of inflammatory bowel disease treated with prednisone. Presented on 17 January 2024 for evaluation of dyspnea and hyporexia. Had multiple episodes of vomiting without response to an increased prednisone dose. He was hospitalized on supplemental O2. Exam revealed a grade 4/6 heart murmur, he was preemptively treated with Vetmedin/Lasix. Radiographs showed evidence of pneumonia. Echocardiogram revealed evidence of mitral and tricuspid valve degeneration with regurgitation, with no left atrial enlargement and no evidence of congestive heart failure. Patient was transitioned to room air on 18 January 2024 and he did well. On the morning of 19 January 2024, patient acutely decompensated and CPR was performed. CPR was successful but patient was euthanized.
Gross Pathology:
Heavy body condition, BCS 7/9, and distended abdomen. Lungs were diffusely wet and heavy, red fluid in the thoracic cavity. The right ventricle was large and dilated, and there were nodular proliferations on the right and left AV valves. No significant findings in the abdominal cavity.
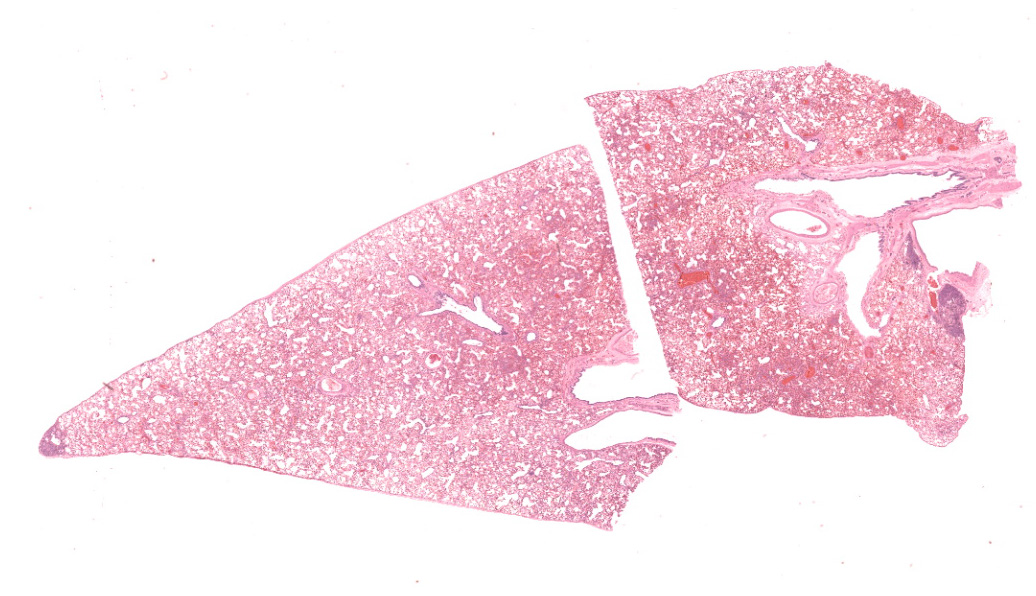
Microscopic Description:
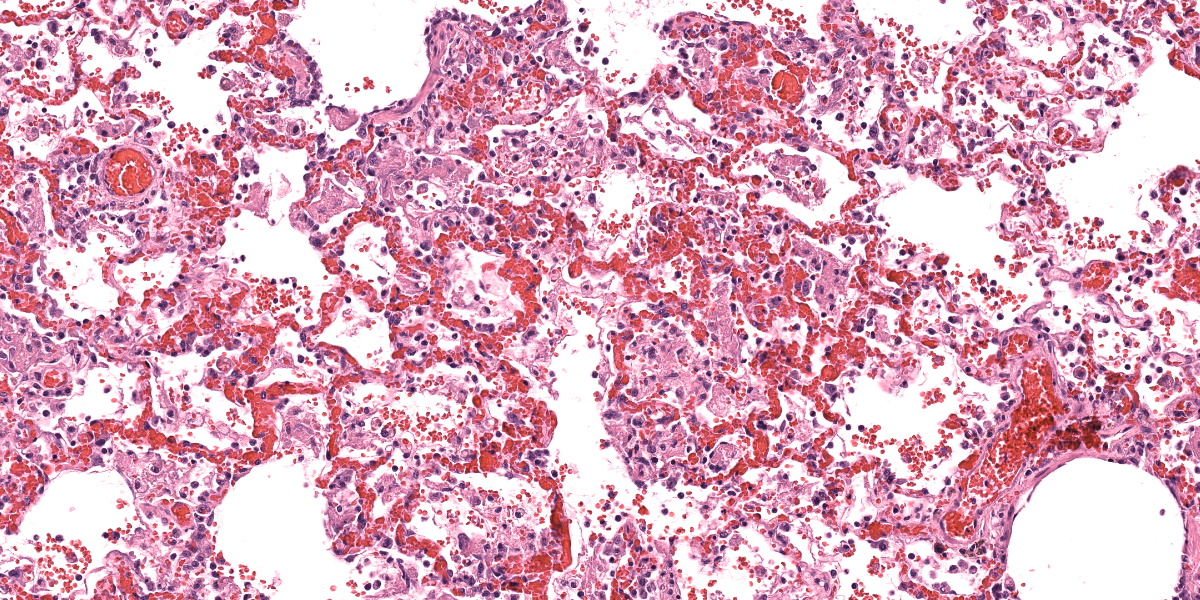
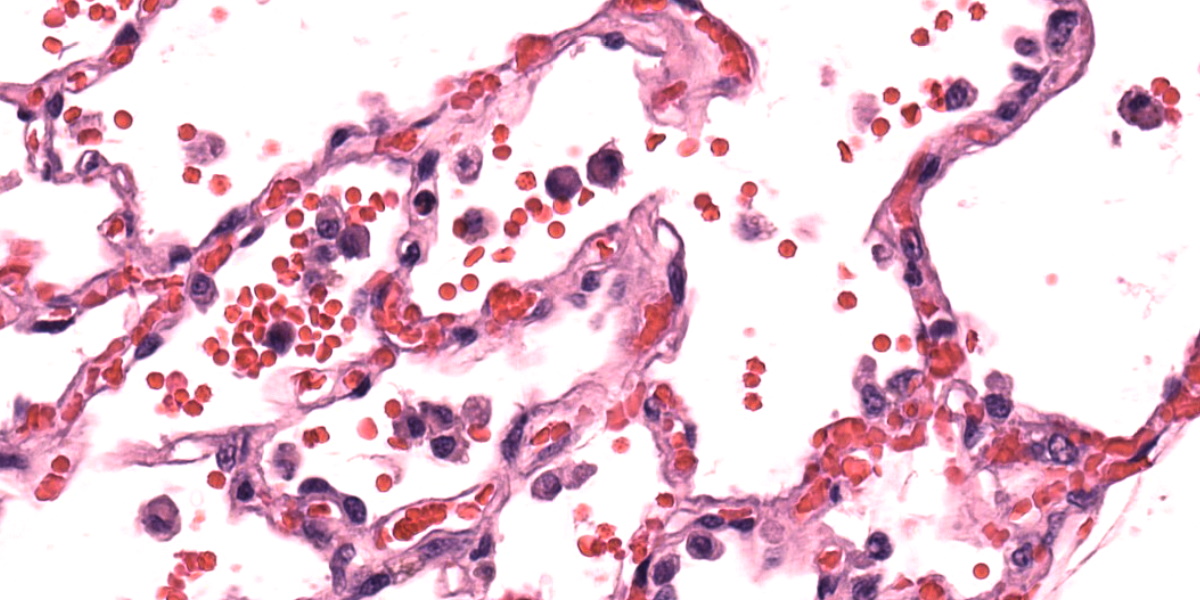
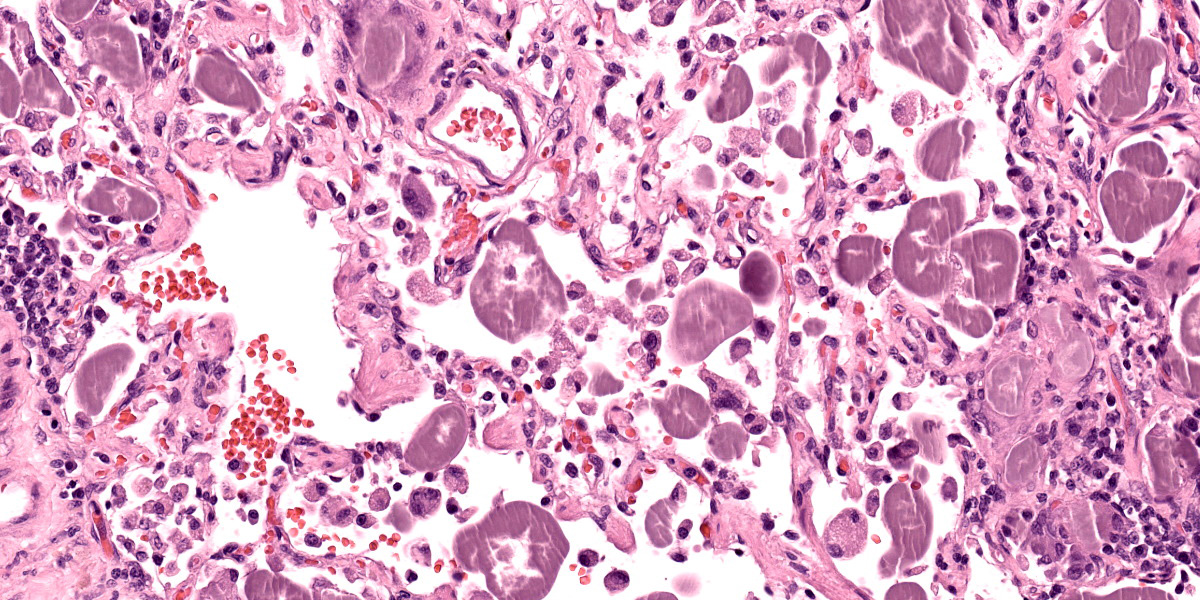
Lungs: Alveolar septa are diffusely thickened by variable combinations and concentrations of fibrin, histiocytes, fewer lymphocytes, edema, and congested alveolar capillaries. Multifocally affecting approximately 40% of the examined tissue, alveolar septa are lined by lamellae of polymerized eosinophilic hyaline material (hyaline membranes), and in these areas type I pneumocytes are often lost or necrotic with nuclear pyknosis or karyorrhexis. Diffusely, alveolar spaces contain variable combinations and concentrations of hemorrhage, edema fluid, variably polymerized fibrin, and increased numbers of foamy alveolar macrophages that occasionally contain phagocytosed erythrocytes and rarely contain hints of cytoplasmic hemosiderin. The perivascular tissue surrounding larger vessels is expanded by edema. The adventitia of small and medium veins multifocally is minimally to mildly expanded by increased fibrous connective tissue. Focally extensively affecting approximately 5% of the examined tissue, adjacent to a bronchus and pulmonary vein, alveoli are filled and expanded by extracellular and intrahistiocytic amorphous, homogeneous, amphophilic, anisotropic, aggregates of hyaline material that vary in size from 5-300 in diameter. The material is multifocally surrounded by granulomatous inflammation composed of foamy macrophages, multinucleate giant cells, and few lymphocytes and plasma cells. At the tip of the margin of one tissue section, there is a focal area of moderate to marked alveolar septal expansion by fibrosis. In this area, there is marked type II pneumocyte hyperplasia, and alveoli contain high numbers of entrapped foamy alveolar macrophages.
The intra-alveolar, hyaline, amphophilic material is strongly PAS-positive.
Not submitted for WSC, the liver had evidence of chronic passive congestion to include central vein adventitial fibrosis, centrilobular congestion, and centrilobular hepatocyte atrophy.
Contributor’s Morphologic Diagnosis:
- Lung: Pneumonia, interstitial, acute, necrotizing, multifocal to coalescing, moderate, with hyaline membrane formation
- Lung: Congestion, diffuse, moderate, with increased alveolar macrophages and perivascular edema
- Lung: Pneumonia, granulomatous, focal, mild, with intraalveolar, extracellular and intrahistiocytic, amphophilic, homogeneous, hyaline material
Contributor’s Comment:
This case represents three pulmonary entities: acute respiratory distress syndrome, chronic passive congestion, and pulmonary hyalinosis.
Acute respiratory distress syndrome (ARDS) is the clinical manifestation of diffuse alveolar damage (DAD).4,5,7 DAD is due to injury to type I pneumocytes and/or alveolar capillary endothelium which results in serum leakage into alveoli, with subsequent pulmonary edema and polymerization of fibrin into characteristic hyaline membranes lining alveolar septa, and eventually type II pneumocyte hyperplasia and interstitial fibrosis.5,7 The pathophysiology of ARDS/DAD is beautifully illustrated in Robbins and Cotran Pathologic Basis of Disease 10th Ed. Figure 15.3.5 ARDS has garnered widespread attention in recent years because the pathogenesis of COVID-19-induced pneumonia is similar.7 Clinically, animals affected with ARDS have acute-onset dyspnea, tachypnea, variable coughing, and lethargy that is often fatal within 3 days.4 The key histologic feature of this condition is the formation of hyaline membranes; these are variably thick, eosinophilic membranes lining alveolar septa, which physiologically prevent gas diffusion leading to respiratory distress.4 Histologically there is also often necrosis or attenuation of terminal bronchiolar epithelium.4,7 DAD may be due to direct or indirect damage, such as the result of multiple organ dysfunction syndrome.4,7 Notable causes of diffuse alveolar damage include smoke inhalation, oxygen toxicity, inhalation of toxic gases (ammonia, phosgene, ozone, etc.), toxin ingestion (paraquat, 3-methylindole, perilla mint, etc.), near drowning, strangulation, septicemia, shock, massive trauma, and chronic left sided heart failure, although many cases are idiopathic.4,5,7
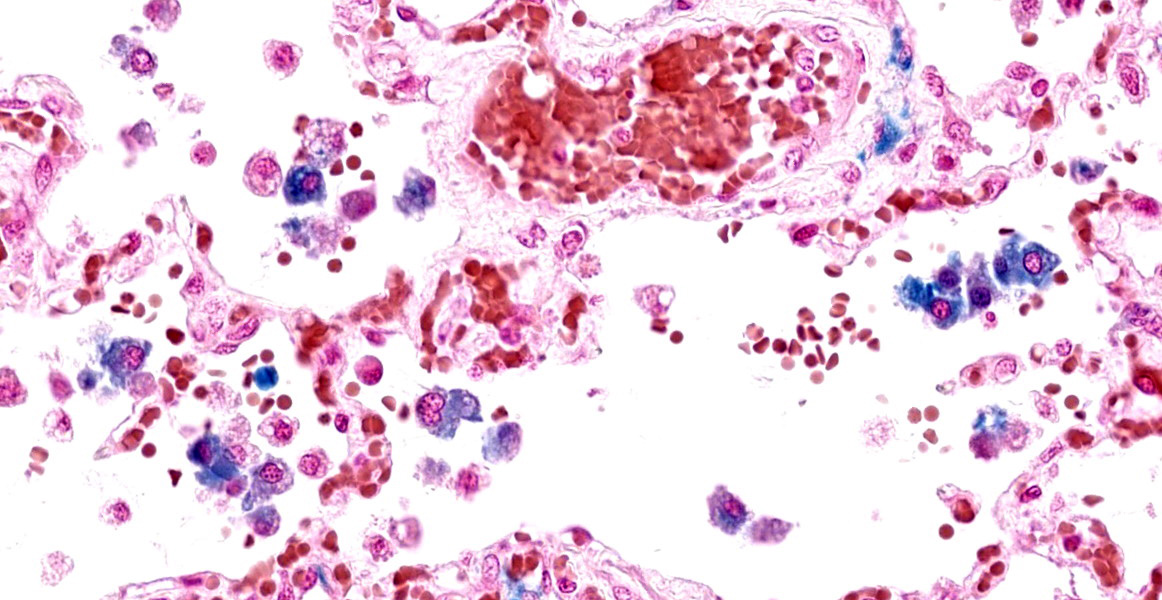
In humans, congestive left-sided heart failure with subsequent pulmonary congestion often results in accumulation of so-called “heart failure cells” within alveoli.6 These “heart failure cells”, also termed siderophages, are simply hemosiderin-laden alveolar macrophages that have phagocytosed red blood cells.4,6,7 In veterinary medicine (with the exception of non-human primates), it is rare to see classic “heart failure cells” associated with chronic heart failure; however, the number of non-hemosiderin-laden alveolar macrophages is often increased secondary to chronic pulmonary edema.4 Additionally of note, hemosiderin-laden alveolar macrophages can be present in conditions other than heart failure, such as pulmonary hemorrhage.4 Pulmonary sequelae to chronic left-sided heart failure are pulmonary edema, increased alveolar macrophages, and pulmonary vein remodeling.4,7 The pulmonary vein remodeling may simply consist of subtly increased adventitial collagen of small to medium-sized pulmonary veins.4
Pulmonary hyalinosis is an uncommon, idiopathic condition in older dogs that is due to an uncharacterized alveolar-filling disorder.7,8 Alveolar-filling disorders are characterized by accumulations of abnormal material in alveoli, and include alveolar histiocytosis, endogenous lipid pneumonia, alveolar proteinosis, alveolar phospholipidosis, alveolar microlithiasis, and pulmonary hyalinosis.7,8 Pulmonary hyalinosis is considered an incidental finding.4,7,8 The material within alveolar lumina is characteristically amphophilic, amorphous or lamellar, birefringent, and strongly PAS-positive.4,7,8 This condition was relatively recently reported in 6 captive sugar gliders.8 This condition was also recently highlighted in the JPC Wednesday Slide Conference (see Conference 5, Case 4, 2022-2023).
Contributing Institution:
Tri-Service Research Laboratory
4141 Petroleum Dr
San Antonio, TX 78234
JPC Diagnosis:
- Lung: Pneumonia, interstitial and organizing, necrotizing and fibrinous, subacute, multifocal, moderate with type II pneumocyte hyperplasia.
- Lung: Congestion, chronic, diffuse, mild to moderate with alveolar siderophages and edema.
- Lung: Pneumonia, interstitial, granulomatous, chronic, multifocal, moderate, with abundant hyaline material.
- Lung: Pneumonitis, lymphocytic, chronic, focal, marked (subpleural alveolar proteinosis).
JPC Comment:
Case 2 showcases multiple entities occurring simultaneously in the lung of an aged dog. Conference participants collectively were able to spot five distinct processes, which reinforces the value of complete slide examination. We did not add a morphologic diagnosis for anthracosilicosis in this case, as it is a common and incidental finding in aged animals, and of a relatively minor degree in this case. Likewise, the pulmonary hyalinosis in this case was not likely significant in the death of this animal. We added a separate morphologic diagnosis for the subpleural alveolar proteinosis, which is a common incidental finding in older dogs, but is worth noting.
The group differed from the contributor in their interpretation of hyaline membranes in this case. Participants noted that the polymerized fibrin filled the alveoli as “fibrin ball”1,3 as opposed to a hyaline membrane, which is typically adherent to the basement membrane itself and visually lines the affected septa.. Likewise, the concurrent type II pneumocyte hyperplasia in this case and increased number of myofibroblasts highlighted by a smooth muscle actin IHC was consistent with at least a subacute course of disease in this animal. Dr Bearss noted that he has seen this phenotype in monkeys utilized for Nipah virus research and drew parallels from his experience
Acute fibrinous and organizing pneumonias (AFOP) are a distinct entity in human medicine1,2,3 that represent an acute to subacute phenotype of severe lung injury. With this slower onset, the mix of plasma protein, surfactant, fibrin, and cellular debris characteristic of a classic acute hyaline membrane is infiltrated by inflammatory cells and embedded myofibroblasts and forms a distinct “fibrin ball” in the alveolus. Likewise, this ‘slow burn’ also provides time for pneumocyte hyperplasia that prevents direct adhesion of material to the basement membrane. In time, this plug of material may also trap inhaled air and lead to secondary emphysema and/or bronchiectasis.1,3. Whether or not left-sided heart failure in conjunction with oxygen therapy (and generation of radical oxygen species) was sufficient to damage the endothelium and/or type I pneumocytes was not resolved in the conference discussion. Herein we use ‘fibrinous’ and ‘organizing’ to capture the nature and distribution of this material and introduce this term to a wider audience.
Finally, conference participants touched on the heart failure aspect of this case. Hemosiderin-laden macrophages were a more subtle pickup in this case absent the history, though some participants also noted erythrophagocytosis which was another confirmation of chronic congestion and stasis of pulmonary blood flow. Alveolar macrophages stained strongly with Perl’s Prussian blue, confirming the presence of iron.
References:
- Al-Khouzaie TH, Dawamneh MF, Hazmi AM. Acute fibrinous and organizing pneumonia. Ann Saudi Med. 2013 May-Jun;33(3):301-3.
- Baque-Juston M, Pellegrin A, Leroy S, Marquette CH, Padovani B. Organizing pneumonia: what is it? A conceptual approach and pictorial review. Diagn Interv Imaging. 2014 Sep;95(9):771-7.
- Bharti JN, Satyendra BT, Shekhawat RS, Gorchiya A. Acute Fibrinous and Organizing Pneumonia—A Rare Lung Pathology. The American Journal of Forensic Medicine and Pathology 43(1):p e1-e3, March 2022.
- Caswell JL, Williams KJ. Respiratory System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 2. 6th ed. St. Louis, MO: Elsevier; 2016: 485, 489, 493, 509-511, 514, 517, 518, 519.
- Husain AN. The lung. In: Kumar V, Abbas AK, Fausto N, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 10th ed. Philadelphia, PA: Elsevier; 2021:676-678.
- Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 10th ed. Philadelphia, PA: Elsevier; 2021:118.
- López A, Martinson SA. Respiratory System, Thoracic Cavities, Mediastinum, and Pleurae. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 7th ed. St. Louis, MO: Elsevier; 2022:571, 574-575, 577-578, 588, 638.
- Sokol SA, Agnew DW, Lewis AD, Southard TL, Miller AD. Pulmonary hyalinosis in captive sugar gliders (Petaurus breviceps). J Vet Diagn Invest. 2017;29(5):691-695.