Signalment:
Gross Description:
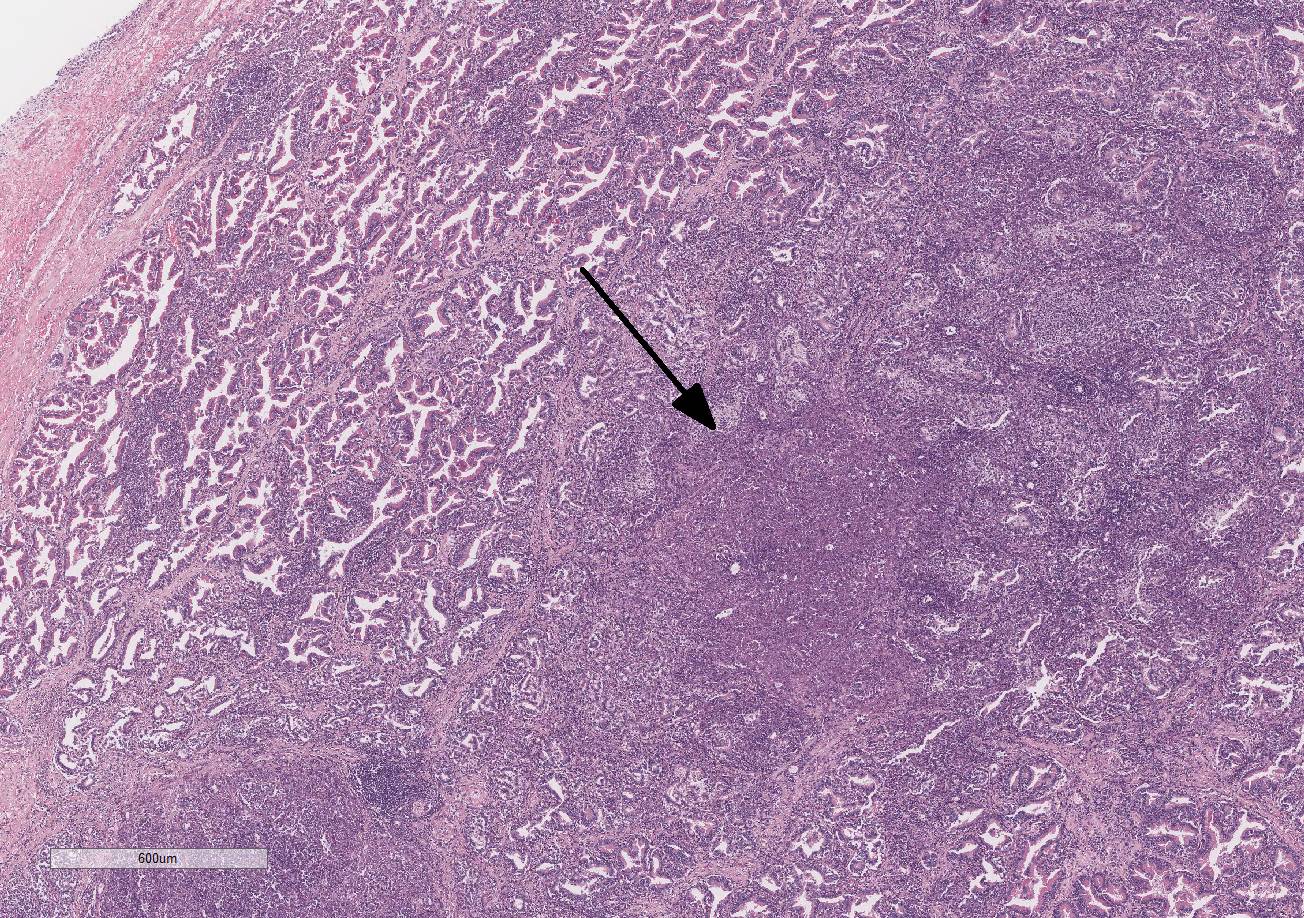
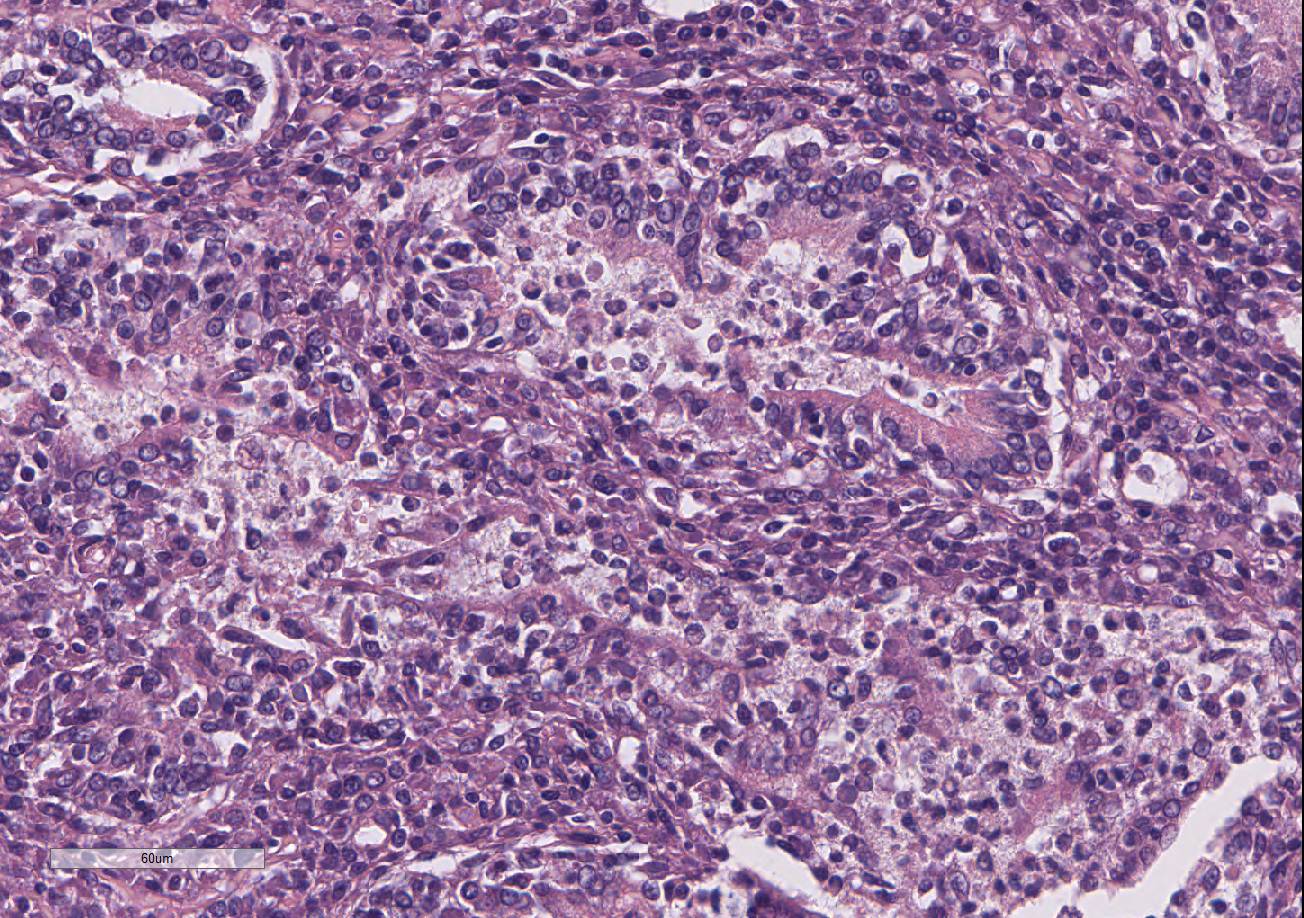
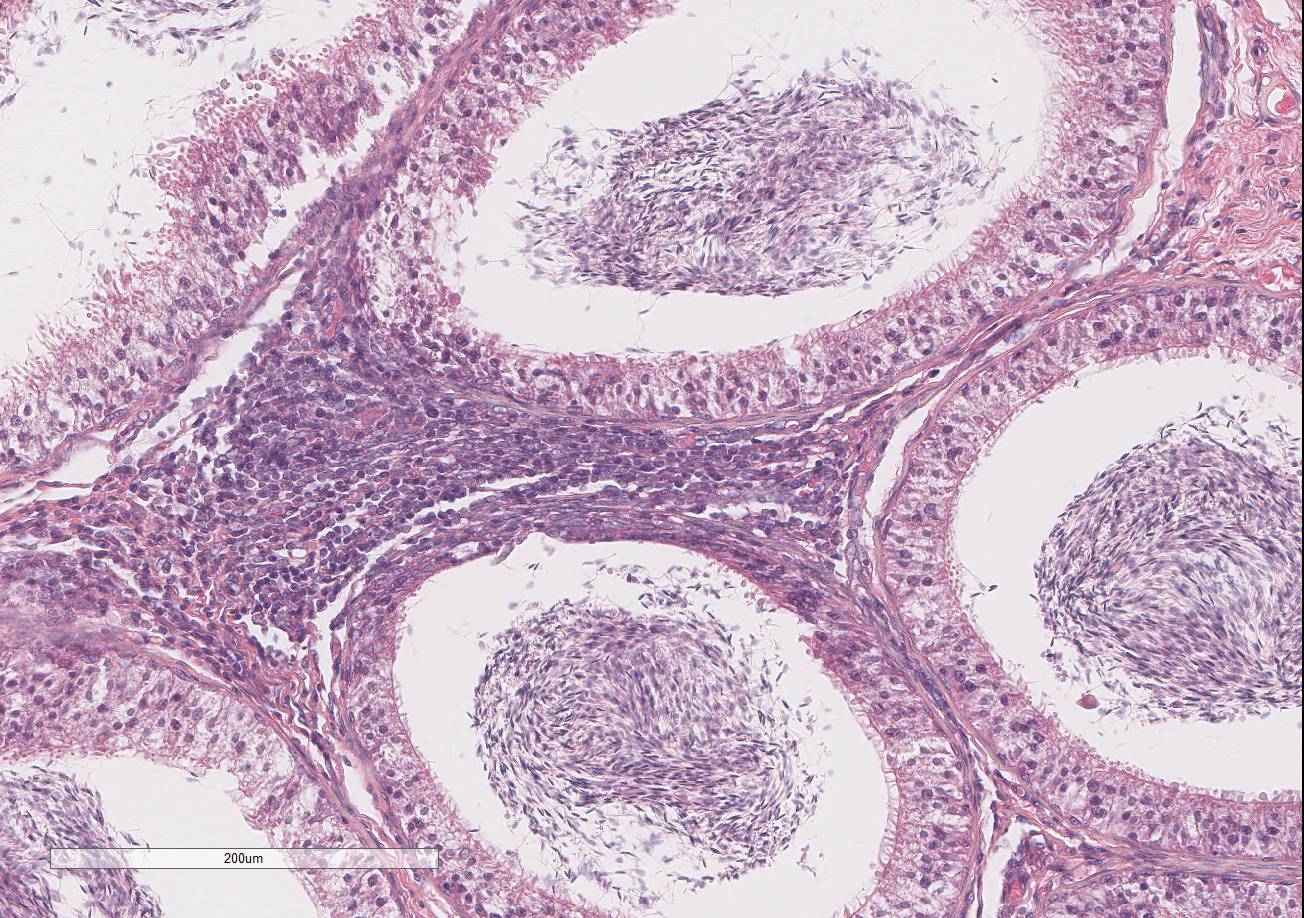
Histopathologic Description:
Testis and epididymis: There is multifocal infiltration of the epididymal connective tissue stroma by small clusters of lympho-cytes, plasma cells, and few macro-phages with a perivascular to random distribution. The ducts contain a moderate amount of sperm. In the testis, there is a moderate to severe decrease in complete spermiogenesis, with many tubules lacking luminal spermatozoa. Gram staining is negative for organisms.
Morphologic Diagnosis:
2. Epididymis: Epididymitis, lympho-plasmacytic, multifocal, chronic, moderate.
Lab Results:
Condition:
Contributor Comment:
Infection occurs via bacterial penetration of mucus membranes, especially oral, conjunctival, and genital. Semen and vaginal fluids of infected canines have very high bacterial loads and exposure to these fluids is the most common route of mucosal transmission, with exposure to infected aborted fetuses, placental tissues, lochia, and urine (especially from male dogs) also serving as sources of infection.4,12 In addition, B. canis has also been isolated from saliva, nasal and ocular secretions, milk, and feces, but the importance of these as sources of infection is unknown.12
Upon penetration of mucus membranes, the bacteria are taken up by phagocytes and trafficked to lymphatic organs and the genital tract. Brucella canis can persist intracellularly by evading phagosome-lysosome fusion and replicate within an endoplasmic reticulum-derived vacuole.2,11 Bacteremia develops 1 to 4 weeks post infection and can persist, intermittently, for several years.4,11
Infected canids often show vague or non-specific clinical signs, including poor hair coat, lethargy, weight loss, back pain, lymphadenomegaly, and reproductive failure.5 In bitches, there may be infertility, early embryonic death, fetal resorption, and late term abortion (45 to 60 days).4,6 Aborted fetuses are born dead and partially autolyzed with subcutaneous congestion and edema.12 Pups can be born alive but usually die shortly after birth. Brown to green vaginal discharge can occur for one to six weeks post abortion. In males, Brucella sp. can cause infertility, epididymitis, prostatitis, scrotal dermatitis (secondary to licking trauma), testicular swelling or atrophy, and loss of libido.4 Sterility can occur secondary to testicular damage that may result in autoimmune anti-sperm antibodies.4,12 Less commonly, in both sexes infection can result in uveitis, diskospondylosis of the thoracic and lumbar spine, glomerulonephritis, and very rarely meningoencephalitis.
Gross lesions of brucellosis may be lacking or vague, often limited to splenomegaly, lymphadenomegaly, and in males, an enlarged epididymis. In the uterus, Brucella species cause chronic to subacute endo-metritis with glandular hyperplasia and reticular cell nodules.4 In males, classic histologic findings are similar to what is presented in this case: lymphoplasmacytic interstitial epididymitis and prostatitis. Other findings can include focal hepatic necrosis, myocarditis, meningoencephalitis, hyaline thickening of the basement membrane of glomeruli, and granulomatous uveitis with retinitis.4
The diagnosis of brucellosis can be made based on a history of reproductive failure and supportive serology with positive blood culture.6 There is no effective treatment for B. canis. Treatment can eliminate active bacteremia but cannot eliminate bacteria residing in tissues; thus, infected tissues serve as sources for recurrent bacteremia.12 Spaying and neutering infected dogs can help decrease shedding of the bacteria but does not eliminate infection.
Brucella canis is a zoonotic agent, but its significance as a human pathogen is poorly understood. Confirmed transmission to humans is not common, although it is likely that the disease is under-diagnosed.4,6 Veterinarians, kennel workers, and people living with a B. canis-positive dog are at greatest risk of infection. In humans, Brucella causes vague clinical signs of fever, weakness, headache, joint pain, and enlarged lymph nodes;12 it has also been associated with ocular lesions and endocarditis.4,6 It is of greatest risk to immunosuppressed individuals, children, and pregnant women. In all cases, counseling about the potential health risks of living with a B. canis-positive dog should be provided.
JPC Diagnosis:
2. Prostate gland: Hyperplasia, cystic, glandular, diffuse, moderate.
3. Epididymis: Epididymitis, lymphocytic, multifocal, mild.
Conference Comment:
Brucella species consist of both smooth and rough strains, with rough strains lacking the expression of the virulence factor O-side chain on the lipopolysaccharide (LPS) present on smooth strains.8 The smooth strain LPS is non-endotoxic and allows entry into host cells via cholesterol-rich lipid rafts in the plasma membrane. In addition, the O-side chain prevents complement-mediated bacterial lysis and inhibits apoptosis of the infected cell. Both smooth and rough strains are adept at survival within macrophages due to expression of the VirB operon encoded type IV secretion system, which is induced by acidification of the phagosome during the respiratory burst. The VirB system neutralizes the pH of the phagosome and allows Brucella species to undergo intracellular replication and survival. Virulent strains also employ other methods to detoxify free radicals within the phagosome, including expression of superoxide dismutates. Intracellular survival and replication is the key to its virulence, and once infection is established, it tends to persist.2,3,9,13
Rough strain Brucella species are phago-cytosed following recognition by toll-like receptor-4 (TLR-4) and are less virulent due to their lack of LPS O-side chain; however, as mentioned above, both have the ability to survive within phagocytes via the VirB operon.3,9 Brucella ovis and B. canis are rough strain groups, while B. abortus, B. melitensis, and B. suis are more virulent and categorized under smooth strains.3,7,9
Conference participants discussed the emergence of zoonotic B. canis in underserved communities worldwide due to human interaction with populations of free-roaming canines. While B. canis is classified as a rough strain, and thus has a lower virulence, there have been sporadic reports of human infection. The incidence is likely under-reported due to its non-specific symptoms of low grade fever, joint pain, headache, and fatigue.1,7
References:
2. De Figueiredo P, Ficht TA, Rice-Ficht A, Rossetti CA, Adams LG: Pathogenesis and immunobiology of brucellosis: review of Brucella-host interactions. Am J Pathol. 2015; 185(6):1505-17.
3. De la Cuesta-Zuluaga JJ, et al. Identification of the virB operon genese encoding the type IV secretion system, in Columbian Brucella canis isolates. Vet Microbiol. 2013. 163:196-199.
4. Greene CE, Carmichael LE: Canine Brucellosis. In: Green CE, ed. Infectious diseases of the dog and cat. 4th ed. St. Louis, Mo.: Elsevier/Saunders; 2012:398-411.
5. Hollett RB: Brucellosis. In: Ettinger SJ, Feldman EC, eds. Textbook of veterinary internal medicine : diseases of the dog and the cat. 7th ed. St. Louis, Mo.: Elsevier Saunders; 2010: 882-6.
6. Hollett RB: Canine brucellosis: outbreaks and compliance. Theriogenology. 2006;66(3):575-87.
7. Kazmierczak J. Public health implication of Brucella cais infection in humans. Summary findings of Brucella canis Workgroup. March 2012. Available at: http://www.nasphv.org/Documents/BrucellaCanisInHumans.pdf. Accessed 6 January 2017.
8. Larsen AK, Nymo IH, Boysen P. et al. Entry and elimination of marine mammal Brucella spp. by hooded seal (Cystophora cristata) alveolar macrophages in vitro. PLoS One. 2013; 8: e70186. doi: 10.1371.
9. Olsen SC, Palmer MV. Advancement of knowledge of Brucella over the past 50 years. Vet Pathol. 2014; 51(6):1076-1089.
10. Schlafer DH, Foster RA. Female genital system. In: Maxie MG ed. In: Jubb Kennedy and Palmer's Pathology of Domestic Animals. Vol 3. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016:402-406.
11. Von Bargen K, Gorvel JP, Salcedo SP: Internal affairs: investigating the Brucella intracellular lifestyle. FEMS Microbiol Rev. 2012;36(3):533-62.
12. Wanke MM: Canine brucellosis. Anim Reprod Sci. 2004;82-83:195-207.
13. Zachary JF. Mechanisms of microbial infection. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Mosby Elsevier; 2012:189-90, 197-8.