CASE I: S 962-17 (JPC 4116730)
Signalment:
Seven-month-old, male sheep (Ovis aries)
History:
The sheep was presented due to persistent chronic stridor. Endoscopically, the larynx was severely swollen. Finally, the animal was sacrificed due to acute onset of severe respiratory distress.
Gross Pathology:
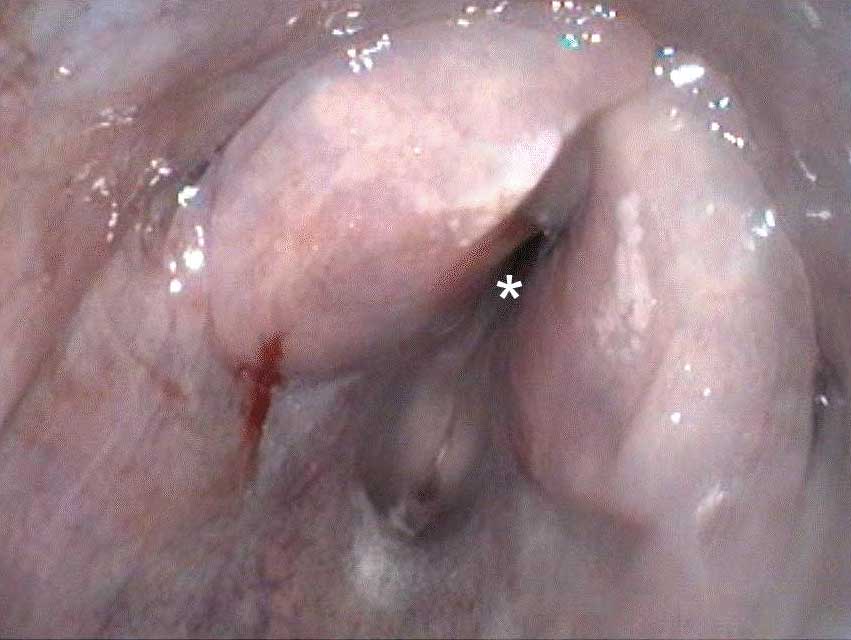
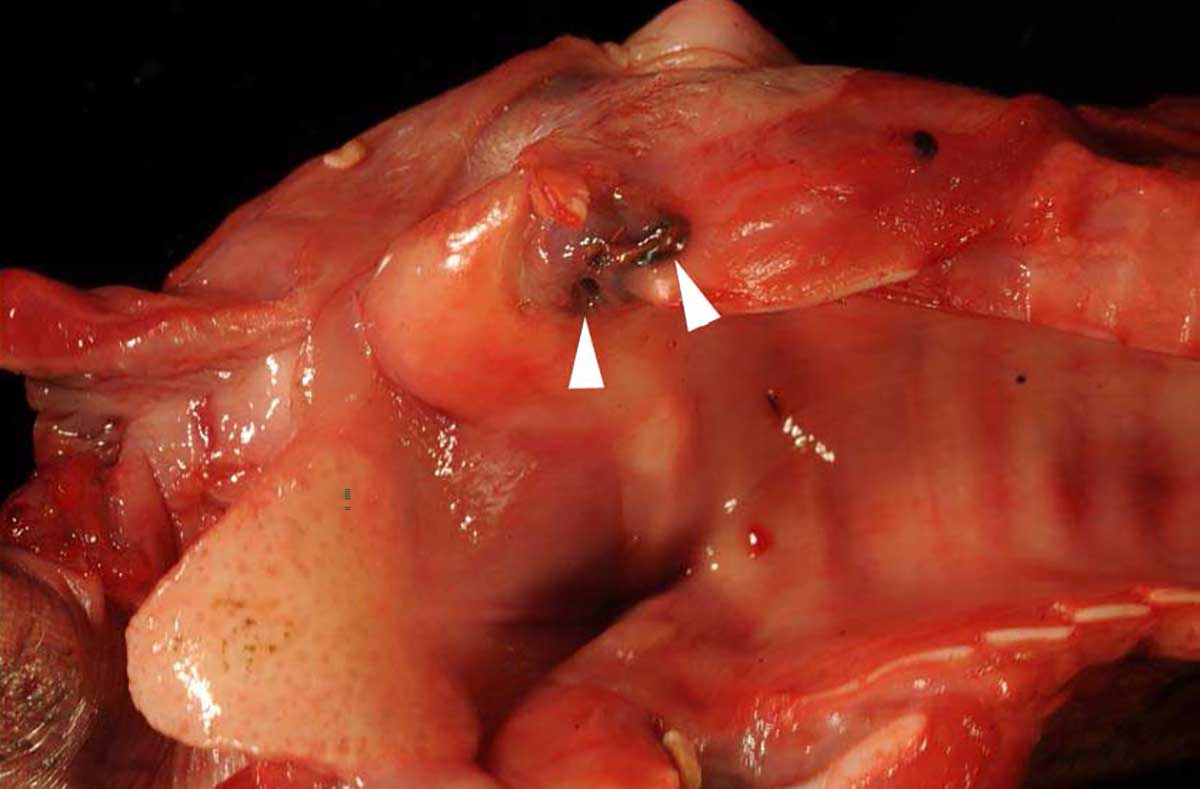
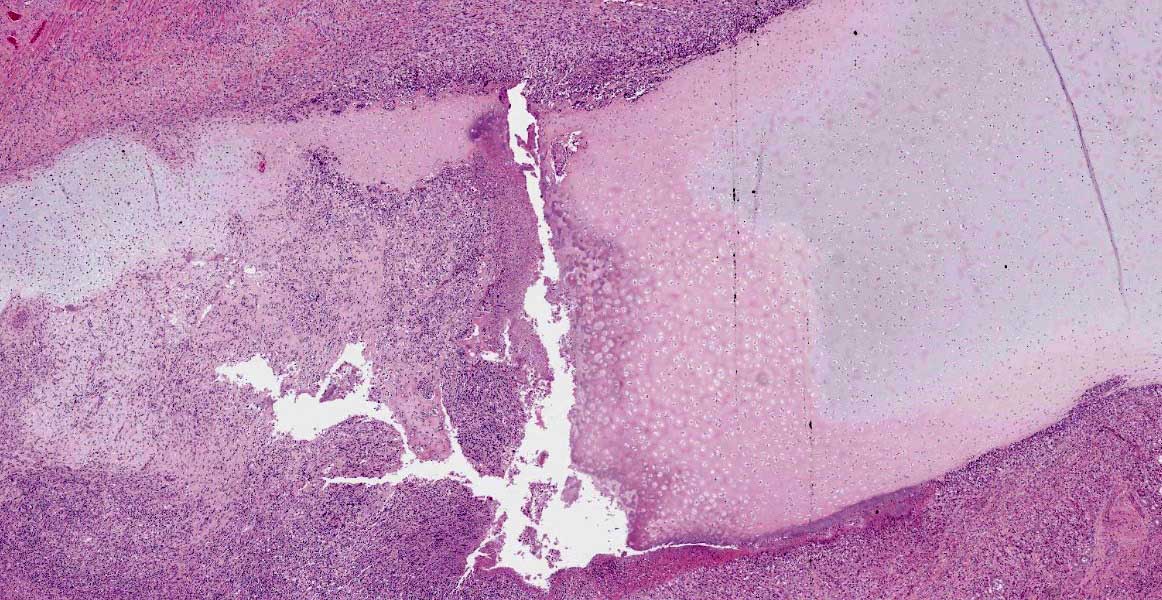
Macroscopically, the larynx exhibited a severe, diffuse edema with obstruction of the laryngeal cavity. Bilaterally, the mucosa of the dorsal parts of the arytenoid cartilages showed approximately 0.5 cm in diameter sized, dark brown, focal defects with extension into the cartilage. Within the cartilage, a 0.5 x 1 x 1 cm large cavity filled with a yellowish, viscous material was present.
Additionally, the right cranial and medial pulmonary lobes displayed a moderate, multifocal, catarrhal and suppurative bronchopneumonia.
Laboratory Results:
Microbiologically, Fusobacterium necrophorum, Streptococcus ovis and Clostridium septicum were isolated from laryngeal tissue.
Microscopic Description:
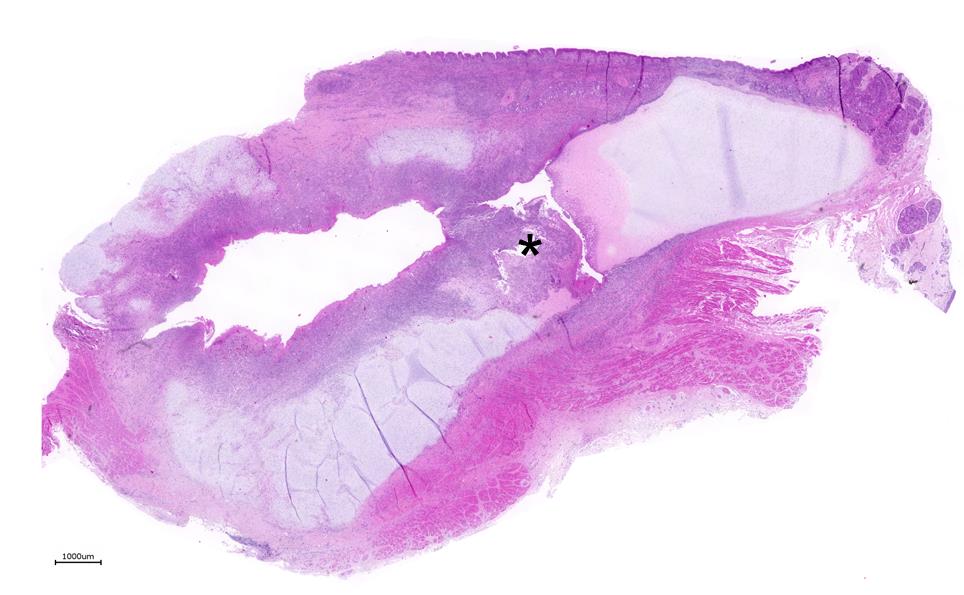
Larynx: Within the arytenoid cartilage a 1 x 0.5 cm sized cavity communicating with the
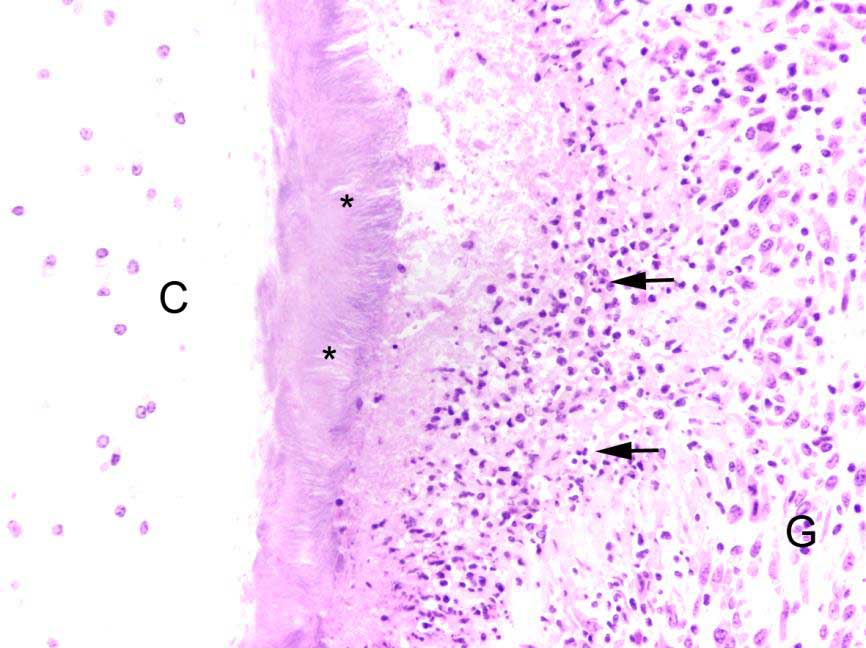
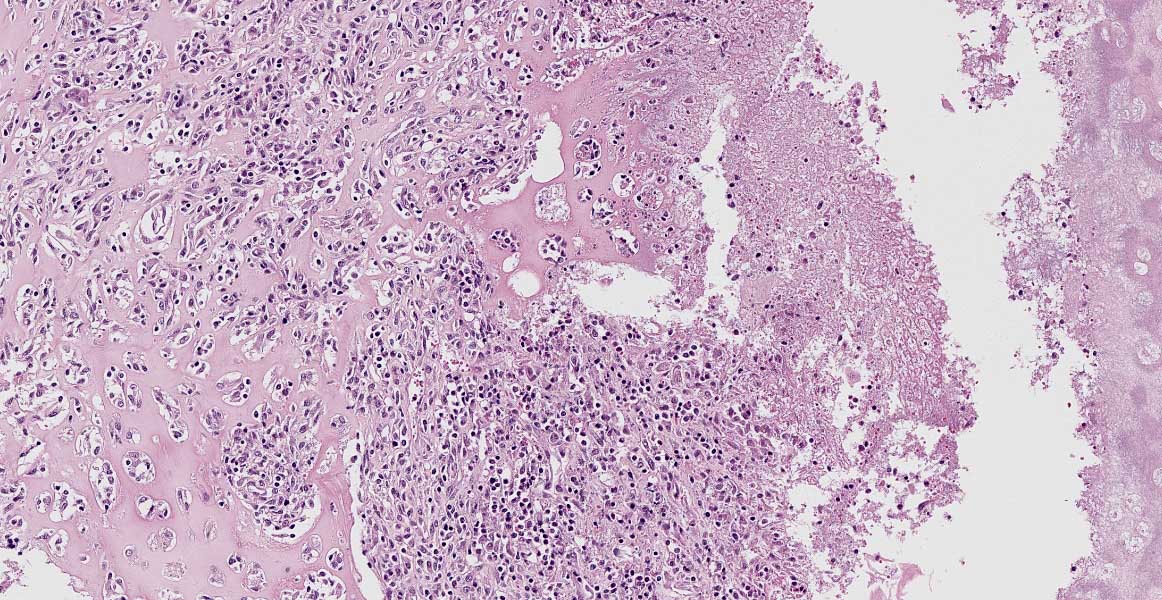
laryngeal cavity was found. This cavity and the surrounding tissue including cartilage, submucosa, submucosal glands and skeletal musculature were infiltrated by numerous inflammatory cells. These infiltrates consisted of high numbers of viable and degenerated neutrophils and fewer macrophages, lymphocytes and plasma cells. Furthermore moderate amounts of cellular debris were found, mainly within the cavitation. The matrix of the adjacent cartilage exhibited a loss of basophilia and numerous shrunken, hypereosinophilic chondrocytes and empty lacunae (necrosis). Additionally, the cartilage surface was irregularly shaped with clefts and fragmentation of the cartilaginous matrix. The luminal surface was lined by high numbers of 0.2 x 7-10 ?m sized basophilic filamentous bacteria and fewer basophilic round cocci of approximately 1 ?m in diameter. Filamentous bacteria were also found within underlying parts of cartilaginous matrix. Furthermore, the intracartilaginous inflammatory infiltrate was multifocally accompanied by moderate numbers of fibroblasts embedded in a collagenous matrix with perpendicularly arranged blood vessels (granulation tissue).
Histochemical analysis revealed a decreased, cartilaginous matrix staining intensity by Alcian blue and Safranin-O in the arytenoid lesions. The Gram stain highlighted gram-positive, coccoid bacteria adjacent to cartilaginous ulcerations. In addition, abundant PAS-positive, gram-negative bacteria arranged in chains were demonstrated at the surface of cartilaginous ulcerations and within the underlying parts of arytenoid cartilage. High amounts of collagenous fibers were detected within the granulation tissue using the Azan stain.
The laryngeal mucosa exhibited multifocal thinning and loss of epithelium with infrequent karyopyknosis, karyorrhexis and karyolysis as well as sloughing of epithelial cells (ulceration and necrosis). Occasionally, the cytoplasm of macrophages contained a coarse yellowish pigment (hemosiderin) or single erythrocytes (erythrophagocytosis).
Contributor?s Morphologic Diagnosis:
1. Larynx: Laryngitis, severe, focally extensive, chronic, erosive to ulcerative and suppurative.
2. Arytenoid cartilage: Chondritis, severe, focally extensive, chronic, necrosuppurative.
Contributor?s Comment:
Inflammatory changes of the larynx often accompany lesions of the upper and lower respiratory tract but they also appear solely.3 Ovine laryngeal chondritis is a rare entity reported in different sheep breeds in California,1,2 Great Britain,10 Iceland,14 and New Zealand.4,7,13 The disease is characterized by low morbidity and high mortalitiy.14 Clinical examination often indicates chronic inflammatory processes in the upper respiratory tract. Lambs and yearlings of both genders can be affected and disease often occurs within the winter term.1,2,14
Microbiological analysis of affected larynges revealed a variable involvement of Fusobacterium necrophorum, Bacteroides spp., Trueperella pyogenes, Streptococcus spp., Pasteurella spp. and Escherichia coli. Most often a mixed growth of gram-positive and gram-negative bacteria was determined.14 Laryngeal lesions are also reported in ovine systemic pasteurellosis.5
The pathogenesis of this entity remains unclear. Primary traumatization of laryngeal mucosa1,2,13 as well as breed predisposition are discussed.10,14 Irritating agents, toxins, coughing as well as aspirated grains may cause primary mucosal damage,2,3,13,14 thereby disrupting the mucosal barrier.
Similar lesions occur in calves,3,9 thoroughbreds,3,6 and humans.11 The lesions are a part of oral cavity necrobacillosis in calves which is caused by Fusobacterium necrophorum, or they are detected in arytenoid chondropathy of racing horses. In both diseases the pathogenesis remains undetermined although mucosal traumatization due to forced respiration and secondary bacterial colonization is discussed.3 In humans, an auto-immune pathogenesis is discussed. Rarely lesions induced by Mycobacterium tuberculosis,11 or those caused by Corynebacterium diphtheriae are observed.8
Contributing Institution:
Department of Pathology
University of Veterinary Medicine Hannover
Buenteweg 17
D-30559 Hannover
Germany
http://www.tiho-hannover.de/kliniken-institute/institute/institut-fuer-pathologie/
JPC Diagnosis:
Larynx: Chondritis, pyogranulomatous, multi-focal to coalescent, with necrosis and chronic-active perilaryngeal myositis.
JPC Comment:
In 1943, Cameron and Britton described a condition affecting stud yearling lambs in California that they identified as ?chronic ovine laryngitis?. Common features included chronic arytenoid cartilage abscesses and laryngeal edema, which often resulted in the suffocation of affected animals.13 As noted by the contributor, the condition now known as ovine laryngeal chondritis has since been described in multiple geographic locations and was recently described for the first time on the European mainland in two German rams in 2020.12
Risk factors for ovine laryngeal chondritis include season, age of the animal, and breed. Although any sex and age may be affected, lambs and yearlings are overrepresented, with the majority of cases occurring during winter. A theory proposed in regard to younger animals being overrepresented is due to the smaller size of their rima glottidis in contrast to adults. In addition, breeds such as the Texel and Southdown are thought to be predisposed, which may be due to variation in the structure of the upper airway in comparison to other breeds. For example, laryngeal narrowing and shortening have been found to be a feature of Texel rams, which also have disproportional enlargement of arytenoid cartilage and epiglottis when compared to Bluefaced Leicesters. Laryngeal edema influenced by male sexual hormones may also contribute toward development of the disease in rams.12
Affected animals may present with variable range of clinical signs depending on severity, including tachypnea with an increased abdominal component, inspiratory and expiratory laryngeal stridor, extension of the head and neck, tachycardia, and cyanotic mucous membranes.12 External palpation may reveal laryngeal enlargement and may also incite transient worsening of dyspnea and/or coughing.12 Cases are often submitted for post-mortem examination with suspicion of pneumonia.13
The mucosa overlying the arytenoid cartilages may either be thickened by diffuse uni- or bilateral asymmetric edematous swelling or thickened with a pale and undulant surface; either may result in nearly complete airway obstruction. On cut surface, the tissue surrounding the arytenoid cartilage is typically edematous, discolored, and often associated with a purulent exudate.13
Common histopathologic features include extensive chronic-active inflammation with granulation tissue formation within the submucosa, which often extends around cartilage and into adjacent striated muscle. The cartilage is often degenerative and eroded by adjacent inflammation, with purulent or fibrinopurulent exudate concentrated at the periphery, frequently with numerous bacteria admixed.13
As noted by the contributor, mixed populations of bacteria are often isolated. One study13 found >80% of cases had mixed gram-positive and gram-negative bacteria, with the most common isolates (each in approximately 50% of cases) being Fusobacterium spp., Trueperella pyogenes and Streptococcus spp.13
Although early cases of ovine laryngeal chondritis have been successfully treated with antibiotics and corticosteroids, these therapies often fail. As a last resort, tracheostomy may be performed as a life-saving measure. However, surviving animals are of limited value given their utilization as breeding stock is not recommended due to the potential heritable component of this disease.12
Conference participants engaged in spirited discussion regarding appropriate morphologic diagnosis terminology. Specific attention was directed toward appropriate utilization of "coalescing" versus "coalescent". Since key components of the morphologic diagnosis (i.e. chronicity, distribution, and severity) describe a noun (e.g. "chrondritis"), use of the adjective "coalescent" may be more appropriate than the historically ubiquitous verb "coalescing".
References:
1. Britton J. Further observations on chronic ovine laryngitis. Cornell Vet. 1945;35:210?213.
2. Cameron H, Britton J. Chronic ovine laryngitis. Cornell Vet. 1943;33:265?268.
3. Caswell JL, Williams KJ. Respiratory System. In: Maxie G, ed. Jubb, Kennedy, and Palmer's pathology of domestic animals. 6th ed., St. Louis: Elsevier, 2016:481?482.
4. Davis G. A sheep mortality survey in Hawke's Bay. N Z Vet J. 1974;22:39?42.
5. Dyson DA, Gilmour NJ, Angus KW. Ovine systemic pasteurellosis caused by Pasteurella haemolytica biotype T. J Med Microbiol. 1981;14:89?95.
6. Garrett K, Embertson R, Woodie J, Cheetham J. Ultrasound features of arytenoid chondritis in Thoroughbred horses. Equine Vet J. 2013;45:598?603.
7. Gill JM. Diseases of East Friesian sheep. Surveillance. 1999;26:6?7.
8. Hadfield TL, McEvoy P, Polotsky Y, Tzinserling VA, Yakovlev AA. The pathology of diphtheria. J Infect Dis. 2000;181 Suppl 1:S116?120.
9. Jensen R, Lauerman L, England J, et al. Laryngeal diphtheria and papillomatosis in feedlot cattle. Vet Pathol. 1981;18:143?150.
10. Lane JG, Brown PJ, Lancaster ML, Todd JN. Laryngeal chondritis in Texel sheep. Vet Rec. 1987;121:81?84.
11. Loehrl TA, Smith TL. Inflammatory and granulomatous lesions of the larynx and pharynx. Am J Med. 111:113?117.
12. Reineking W, Punsmann TM, Wagener MG, et al. Laryngeal chondritis as a differential for upper airway diseases in German sheep. Acta Vet Scand. 2020;62(1):12.
13. Salisbury R. Chronic ovine laryngitis. N Z Vet J. 1956;4:144?146.
14. Sigur?ard?ttir ?, J?rundsson E, Fri?riksd?ttir V. Laryngeal chondritis in sheep in Iceland. J Comp Pathol. 2016;155:310-313.