Signalment:
Histopathologic Description:
Pancreas: Rarely the endocrine islets are effaced and the cells are individualized by a homogenous, pale, eosinophilic material (amyloid).
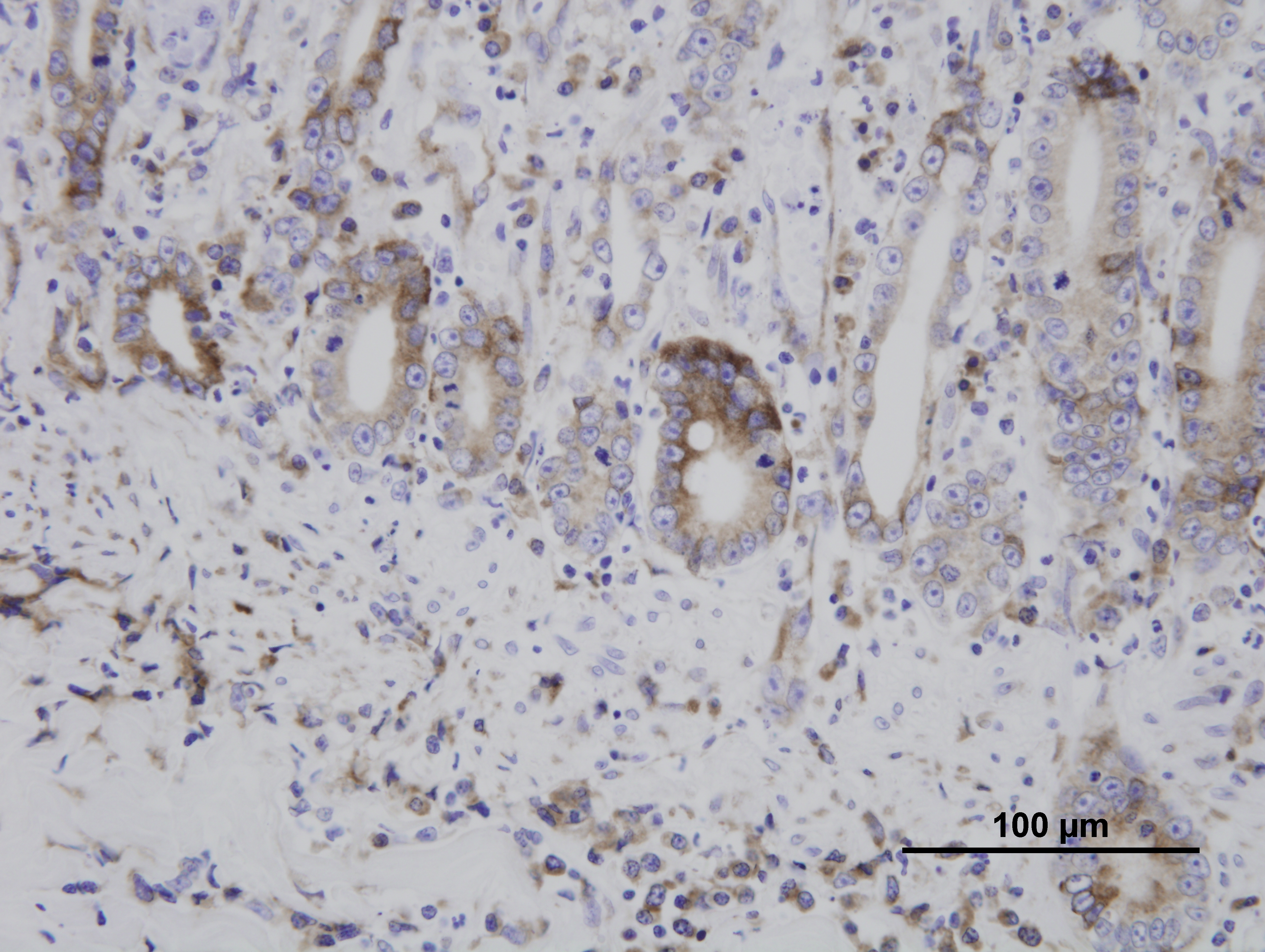
Immunohistochemistry for parvovirus was positive in the duodenum. Immunohistochemistry for Toxoplasma gondii was positive in the duodenum, lung, liver, spleen, and mesenteric lymph nodes. The liver, spleen, and mesenteric lymph nodes are not submitted.
Morphologic Diagnosis:
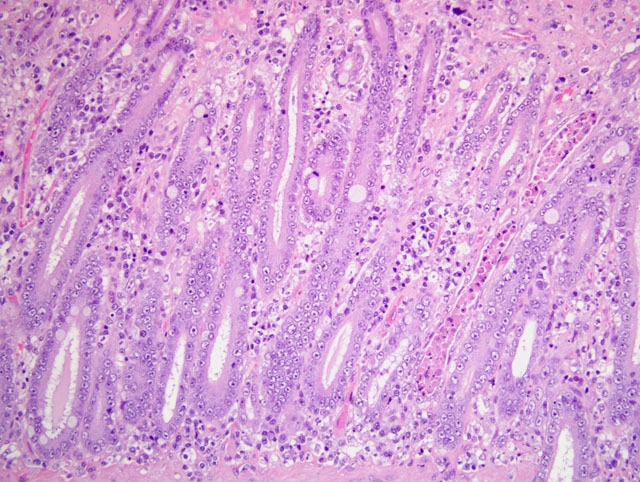
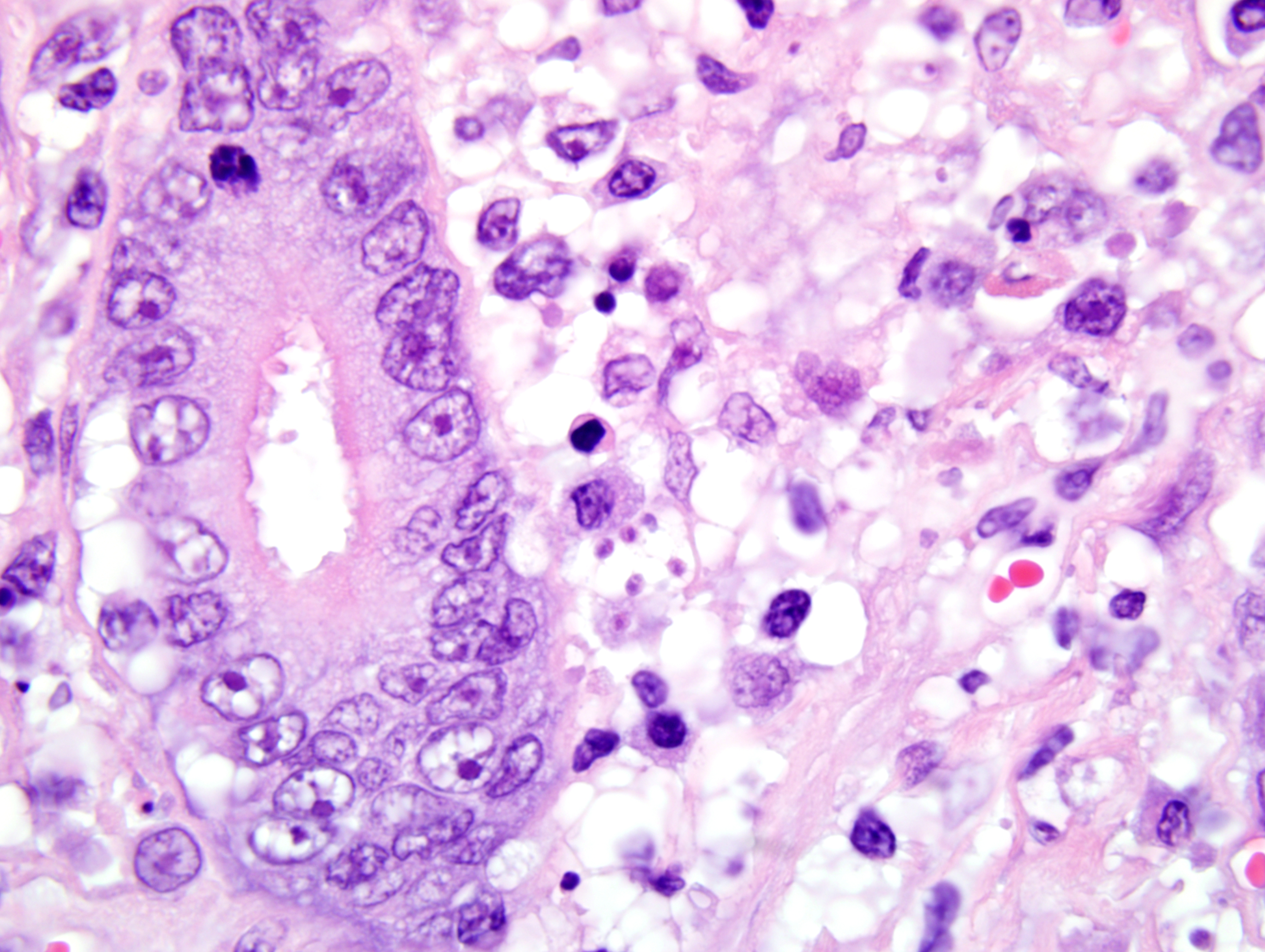
1. Duodenum: duodenitis, lymphohistiocytic, diffuse, with crypt necrosis and crypt abscesses, epithelial loss and regeneration, collapse of lamina propria, and protozoal tachyzoites.
2. Pancreas: amyloidosis, islet, mild.
Condition:
Contributor Comment:
The mode of FPV exposure is typically oronasal, allowing the virus to infect the tonsillar epithelium. Free virus released into the lymph as well as dissemination of infected lymphocytes results in infection of other lymphoid organs, such as the thymus, spleen, Peyers patches, and bone marrow. Lymphocytolysis results in lymphopenia as well as viremia. The enteric system and bone marrow are infected following viremia and by circulating infected lymphocytes. Destruction of crypt cells results in villus atrophy and mucosal erosion or ulceration. Infection of the bone marrow causes depletion of myeloid and erythroid precursors. The lymphopenia and secondary bone marrow destruction can result in significant immunosuppression, allowing the animal to become susceptible to normally harmless infectious agents.
Although infection with Toxoplasma gondii typically results in seroconversion without clinical disease, immunosuppression due to concurrent FPV infection can allow the protozoa to disseminate systemically, resulting in necrotizing lesions in a multitude of organs, such as the liver, heart, spleen, eye, or brain. Cats are easily infected by ingestion of tissues containing the latent asexual stage (bradyzoite) in latently infected prey animals. Another possible but more difficult route of infection of cats is by direct transmission from ingestion of oocysts passed in the feces of another cat (after exposure to oxygen and time for sporulation).
Once Toxoplasma organisms penetrate the intestinal mucosa, the protozoa can spread via lymphocytes to regional lymph nodes, lymph, and the bloodstream, or from the intestine they may pass directly into the portal circulation, allowing dissemination to a variety of organs. Tachyzoites invade (or are phagocytosed by) host cells, proliferate and spread to neighboring cells, causing lysis of host cells upon egress.
JPC Diagnosis:
1. Small intestine, duodenum: Enteritis, necrotizing, diffuse, moderate, with crypt regeneration, few crypt abscesses, and many intracellular and extracellular protozoal tachyzoites.
2. Pancreas, endocrine: Islet amyloidosis, focal, mild.
Conference Comment:
This case is unique in that FPV classically affects younger animals after maternal antibodies have waned. Gross lesions of FPV are most consistently discovered in the thymus (e.g. marked involution and reduced mass in young kittens) and intestines (e.g. dry, nonreflective serosa; mucosal, submucosal, and/or muscular petechiae; and segemental dilation and/or increased turgidity). Because the gross intestinal lesions may be subtle, histopathology is indispensable to achieve a definitive diagnosis of FPV enteritis. Microscopic lesions are most consistently found in the intestines, bone marrow, and lymphoid organs. As noted by the contributor, the rapidly dividing crypt epithelial cells are chiefly affected, and early in the course of infection may contain intranuclear inclusion bodies, particularly within cells sloughed into crypt lumina. The likelihood of visualizing inclusions is increased if tissues are fixed in Bouins solution rather than formalin.(1) Villar enterocytes, which are not actively dividing, are not affected; in the present case, necrosis of villar epithelium is attributed to toxoplasmosis, and crypt regeneration is attributed to FPV.
Because FeLV can cause crypt necrosis in some cats, distinguishing it from FPV depends on examination of the lymphoid and hematopoietic tissues. In early FPV, lymphocytolysis is characteristic in lymphoid organs such as the thymus, lymph nodes, and Peyers patches; by 7-8 days post-infection, regenerative lymphoid hyperplasia may instead be found. Similarly, early bone marrow lesions include marked depletion of all cell line precursors; this results first in neutropenia, because of the relatively short circulating half-life of neutrophils, followed next by lymphopenia, then finally thrombocytopenia. Bone marrow changes later progress to marked stem cell hyperplasia. In FeLV, by contrast, lymphoid hyperplasia, rather than lymphocytolysis, is typical of early disease. Additionally, enteric FeLV is characterized by associated mononuclear cell inflammation in the mucosa, whereas the enteric lesions of FPV infection contain a paucity of inflammatory cells. Antemortem hematology may also be helpful in distinguishing FPV from FeLV; the former elicits panleukopenia, as suggested by its name and as described above, while the latter results in a nonregenerative anemia. Finally, the detection of viral antigen by immunohistochemistry, as employed in this case, is also useful for distinguishing enteric FeLV from FPV.(1)
Conference participants briefly reviewed several other parvoviruses of importance in veterinary medicine, including canine parvovirus (CPV)-2; canine minute virus (CPV-1); mink enteritis virus; Aleutian mink disease virus; bovine parvovirus; porcine parvovirus; and the parvoviruses of rats, mice, and Syrian hamsters. Receptor binding to the transferrin receptor TfR determines the host ranges of FPV and CPV infection.(1)
In most domestic animal species, with the possible exception of placentitis and abortion in sheep and goats, infection with Toxoplasma gondii only rarely causes overt disease. In immunocompetent hosts, cell-mediated immunity, dominated by a TH1 response, is sufficient to maintain the organism as quiescent tissue cysts, which are nonpathogenic. Systemic toxoplasmosis is generally only seen in neonates or immunocompromised hosts, such as the cat in this case. Specifically, decreased levels of interferon-γ, and resultant impaired ability to activate macrophages, are associated with increased susceptibility to systemic disease.(1) During the conference, the moderator drew parallels between this case and canine toxoplasmosis secondary to canine distemper virus infection. For another example of toxoplasmosis and more detailed review of the entity, readers are referred to WSC 2008-2009, Conference 1, case I.
References: