Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 22
April 18th, 2018
CASE IV: #1 (JPC 4101495)
Signalment: 12-year-old, neutered male, European domestic shorthair (Felis catus), feline.
History: Mass developing rapidly in the right mid-thigh. Previous history of multiple antibiotic and vaccine injection on the site were reported by the referring veterinarian.
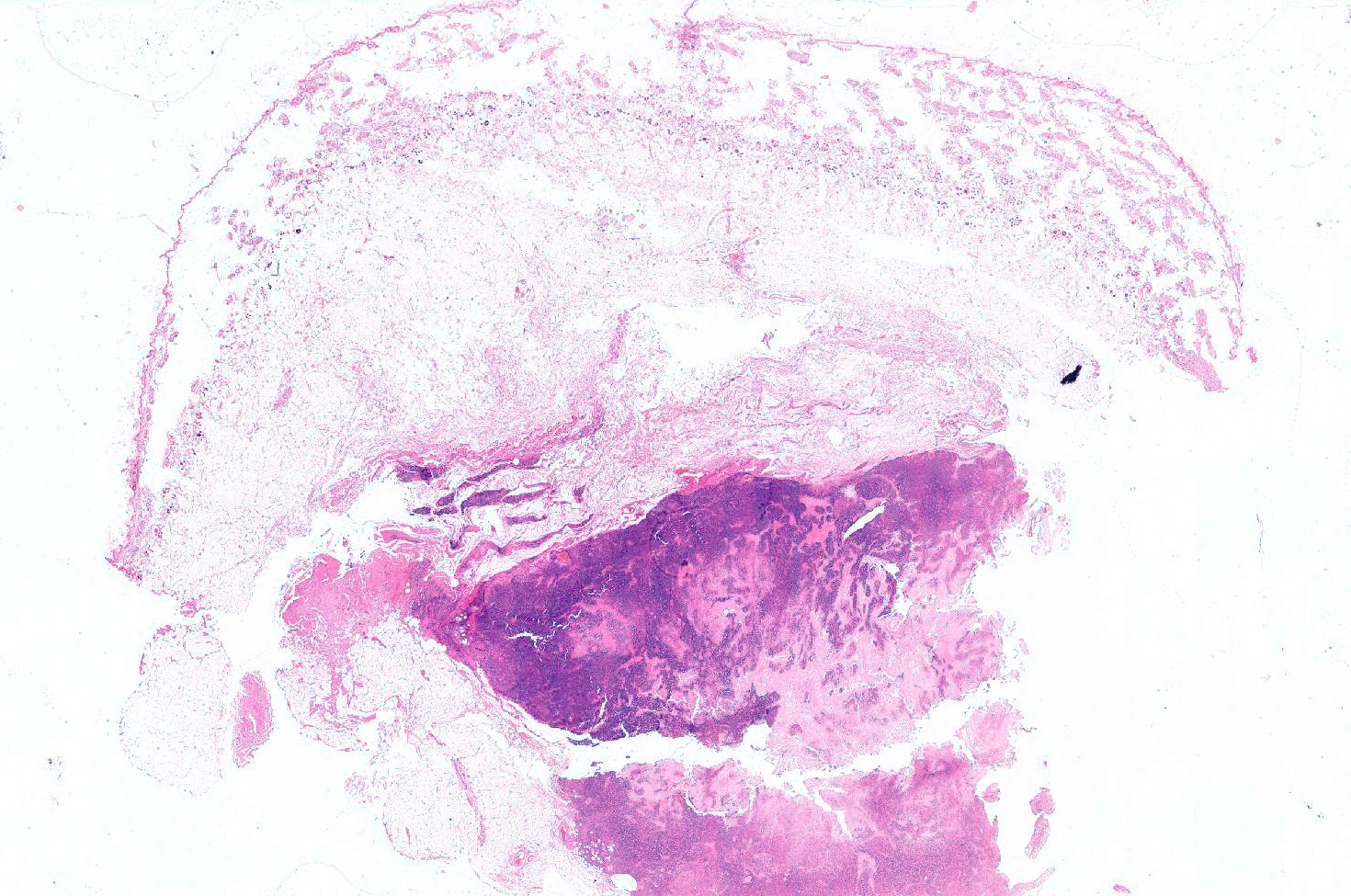
Gross Pathology: Subcutaneous soft mass of large diameter, white tan, blending in the muscle
Laboratory Results (clinical pathology, microbiology, PCR, ELISA, etc.): CBC, Serum biochemistry, urine analysis unremarkable. Clinical staging negative for internal disease.
Microscopic Description:
Haired skin (not always present): The deep dermis, the panniculus and the skeletal muscles are characterized by a variably cellular neoplastic infiltration associated with areas of necrosis. The tumor is not encapsulated, infiltrative, and extending to the borders of the biopsy.
The neoplasm is composed by round neoplastic organized in variably dense sheets or that tightly encircle and invade blood vessels walls (angiocentric and angioinvasive pattern) in association with locally extensive to coalescing areas of necrosis.
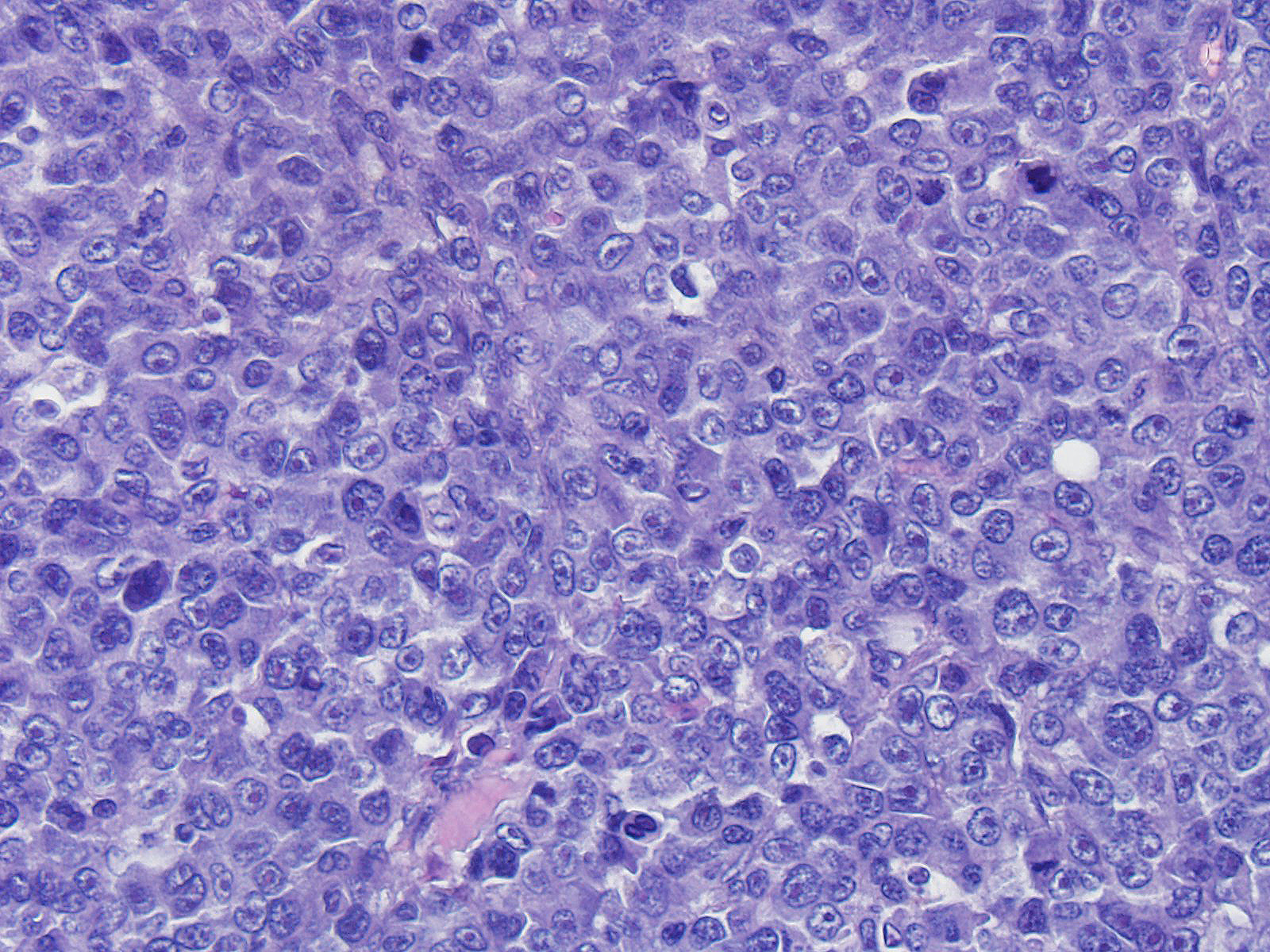
Neoplastic cells range from 20 to 35 microns in diameter, are round, with variably distinct cell borders, high N/C ratio, complete rim of variably eosinophilic homogeneous cytoplasm. Nuclei are round, oval indented, paracentral, 15-30 micron in diameter, with finely granular chromatin and 1 to 4 round, prominent, basophilic nucleoli. Anisocytosis and anisokaryosis are severe. Mitoses are common and range from 2-6 per HPF and are often atypical. Tingible body macrophages range from 1 to 4 per HPF.
Contributor’s Morphologic Diagnosis:
Angiocentric angiodestructive large cell subcutaneous lymphoma (injection site type) with necrosis
Contributor’s Comment: Lymphomas represent more than 50% of all tumors in cats, with a prevalence of approximately 1.6% of the general feline population and 4.7% of hospitalized sick cats.11,23 Primary cutaneous lymphomas account for 0.2 to 3%21 of all feline lymphomas. Cutaneous, non-epitheliotropic lymphomas seem more frequent in cats10 than dogs and include indolent T cell lymphoma, also referred to as cutaneous lymphocytosis,6,7 diffuse T cell lymphoma, T cell rich large B cell lymphoma, and lymphoplasmacytic lymphoma.
In this case, microscopic features parallel descriptions and history of injection site lymphoma in cats.15,17 Primary cutaneous lymphomas developing following injection have been reported in cats.15,17 These lymphomas exhibit several peculiarities, including clinical presentation as a solitary dermal to subcutaneous nodule, development at confirmed previous injection sites (lateral thorax, interscapular region, thighs), microscopical presence of necrosis leading to central cavitation, peripheral inflammation, angiocentric, angioinvasive and angiodestructive growth patterns,15,17 and presence of peripheral inflammation characterized by perivascular nodular lymphoid cell aggregates.17 An additional unusual feature of feline injection site primary cutaneous lymphomas as a group is the prevalence of large B cell lymphomas with centroblastic, immunoblastic and anaplastic morphology17 that are considered rare to exceptional tumors in cats.7,10,21,23
Primary cutaneous diffuse large B cell lymphomas (DLBCL) in man manifest as a solitary nodule or as multiple tumors restricted to one anatomic area (regional disease) and have a relatively poor prognosis compared with other primary cutaneous lymphomas, with a 5-year survival rate of 20-55%.19 The most common morphological variants of human DLBCL are centroblastic, immunoblastic and anaplastic.19 All these features are shared by a large prevalence of feline injection site skin lymphomas.17
The microscopic angiocentric, angioinvasive and angiodestructive pattern described for cutaneous feline injection site lymphomas resembles descriptions of human angiocentric lymphomas (ALs).18 In man, primary cutaneous ALs represent a localized disease with propensity to relapse developing primarily in male patients.18 Distinctively, human ALs are also characterized by extensive tissue necrosis and/or severe inflammation that often obscures the tissue and the neoplastic process itself.16
Pathogenesis of feline cutaneous injection lymphomas may also resemble pathogenesis of human lymphomas emerging in the context of chronic inflammation with transformation of lymphoid cells.4,5,8,9 Chronic inflammation has long been linked to emergence of a wide range of human malignancies and is now generally accepted as a risk factor for development of a variety of cancers including hematopoietic malignancies such as cutaneous T and B cell lymphomas in man.5,8,9 In cats, the development of sarcomas at injections sites (e.g. rabies vaccine, long acting antibiotics or steroids) or at sites of implanted foreign material (non-absorbable suture material, microchip implants, retained surgical sponges or trauma) have been well documented and their pathogenesis has been attributed to the chronic inflammation elicited.12 Progression of chronic inflammation to feline cutaneous lymphoma has also been hypothesized.17 Previously, cutaneous lymphomas have been reported to arise in areas of feline injection site sarcomas following chemotherapy or radiation therapy of the primary tumor, and lymphomagenesis in these cases was hypothesized to derive from the mutagenic action of chemotherapy or radiation treatment.14
Many primary human cutaneous lymphomas including ALs and DLBCL have been consistently associated with inflammation and EBV infection.2,5 DLBCL associated with chronic inflammation (DLBCL-ACI) is a B cell lymphoma included in the WHO classification as a specific entity2 This tumor develops in the context of long standing inflammation associated most frequently with EBV infection.2,4 Most cases of DLBLC-ACI have been described in patients with pyothorax resulting from artificial pneumothorax prescribed for pleural tuberculosis.2 DLBLC-ACI is also angiocentric and, similarly to feline injection site lymphoma, is more frequent in middle aged to old male patients and develops after a long latency period of over 10 years from terminally differentiated B cells.2 In these instances, inflammation has been implicated in the reactivation and proliferation of EBV transformed B cells and seems to be the most accredited pathogenesis for DLBCL-ACI.2 Chronic inflammation enables virally transformed B cells to escape from host immune surveillance trough production of IL-10 and providing autocrine and paracrine cell growth stimuli via IL-6 production. Noteworthy, also for other DLBCL occurring in settings of long standing inflammation such as osteomyelitis, metallic implants, and chronic skin ulceration, EBV positivity of neoplastic cells has been demonstrated. All the above observations parallel the finding of inflammation and concurrent FeLV positivity documented in several cases of CFIL.17 While Feline injection site sarcoma seems not related with a specific viral etiology10,12 the role of FeLV in lymphoma development has been well established.11,13 Overall, approximately 70 percent of cats with lymphoma have FeLV antigenemia. Rate of FeLV serological positivity has been correlated with the anatomical form of lymphoma with percentages of positive cats maximal for mediastinal lymphoma (90%) and multicentric lymphoma (80%) and decreasing to less than 10% for cutaneous lymphoma.11,13
In feline injection site lymphomas, expression of FeLV p27 capsidic and gp70 envelope proteins has been detected in neoplastic cells.13 Expression of p27 indicates that viral infection has occurred but does not confirm viral assembly (nonproductive infection) thus, p27 detection does not imply that FeLV infection is in progress. Gp70 expression denotes viral particle assembly confirming viral integration, viral replication and productive infection.20 In FeLV latent infection, cats are seronegative but FeLV provirus has been demonstrated in peripheral blood and bone marrow cells by PCR. Thus, old seronegative cats may still bear the virus in their genome, but the virus may be inserted and not transcribed until reactivation and/or neoplastic transformation of infected cells occurs. Like what is described for DLBCL-ACI in man, chronic inflammation elicited by the injection may have contributed to FeLV reactivation and transcription with neoplastic transformation of lymphoid cells. The most likely hypothesis linking persistent antigenic stimulation with chronic inflammation and lymphoma development derives from the nature of the lymphoid proliferation. During chronic antigenic stimulation, lymphoid cell proliferation and gene rearrangements of TCR and BCR increase with increasing production of normal cells or cells with genetic mutations or translocations.
JPC Diagnosis: Haired skin (not present on all sections) and subcutis: Lymphoma with angioinvasion, angiodestruction, and coagulative necrosis, European domestic shorthair (Felis catus), feline.
Conference Comment: Throughout the 20th century, lymphomas in domestic animals were classified based on the non-Hodgkin lymphoma classification system in humans. The Rappaport classification, designed in 1966, was one of the earliest systems used in veterinary medicine, especially the dog. This system was based solely on morphologic characteristics (which fallaciously classified many large cell lymphomas as histiocytic). It wasn’t until the advancement of immunohistochemical practices that classification systems were again updated. The Lukes-Collins (North America) and Kiel (Europe) classification systems were published based on immunologic more than morphologic concepts, but often yielded different diagnoses. To unify lymphoma classification, in 1982, the National Cancer Institute initiated a broad study oriented on clinical outcome rather than morphologic features and published the Working Formulation. This classification system was even more unreliable because survival times were based on human clinical trials. Finally, an updated Kiel classification was produced which until recently was the most useful prognostic tool for canine malignant lymphomas.22
The current system used is based on the WHO classification system for hematopoietic neoplasms which has been applied to lymphomas in multiple veterinary species. These classification schemes characterized each type of lymphoma as a specific disease entity. The WHO classification for lymphoma diagnosis in domestic animals entails grouping subtypes based on pattern (diffuse or nodular), cell size, grade, postulated normal cell counterpart, and defining histopathologic features.1,22 Cell size is determined based on the size of a red blood cell (RBC) with large lymphocytes being greater than twice the size of an RBC, intermediate lymphocytes being 1.5 times the size of an RBC, and small lymphocytes being 1 to 1.25 times the size of an RBC. Grade is determined by the mitotic count per 400x field with indolent being 0-1/HPF, low grade being 2-5/HPF, medium 6-10/HPF, and high greater than 10 mitotic figures per 400x HPF. Cell size can be difficult to appreciate, secondarily, chromatin pattern can be used to distinguish between different forms of lymphoma. For example, small cell lymphomas have very dense chromatin and intermediate in a few subtypes have prominent nucleoli (Burkitt-like subtype) whereas others have hazy chromatin with indistinct nucleoli (lymphoblastic lymphomas). Immunohistochemically, there are several available B-cell markers (e.g CD20, CD79a or b, Pax5), and it is optimal to use more than one in order to capture the distinct maturation phases of B-cells. CD3 is an excellent pan-T-cell marker. It is important to note that PARR is a genotyping test for clonality, and must always be run in concurrence with immunophenotyping protocols (e.g. IHC, ICC, flow cytometry).
The moderator briefly discussed cancer arising in an inflammatory background. Inflammation is causally related to cancer via: genotoxicity, aberrant tissue repair, proliferative responses, invasion, and metastasis. For example, STAT3 and NF-kB pathways are involved in diffuse large B-cell lymphoma oncogenesis. Additionally, tumor cells secrete soluble growth factors, and render inflammatory cells suppressive against host immune responses. Finally, some microbial organisms are causative agents of cancer inducing inflammation, and commensal microbiota, if altered, can predispose to neoplastic transformation.3
Unfortunately, the unstained slides submitted contained a different tissue than what was submitted on H&E with the tumor. We were therefore unable to fully subtype the lymphoma in this case.
Contributing Institution:
DIMEVET, Faculty of Veterinary Medicine of Milan, Italy
http://www.dimevet.unimi.it/ecm/home
References:
- Boes KM, Durham AC. Bone Marrow, blood cells, and the lymphoid/lymphatic system. In: Zachary JF, ed. Pathological Basis of Veterinary Disease. 6th ed. Philadelphia, PA: Mosby Elsevier Inc.; 2017:724-803.
- Chan JKC, Aozasa K, Gaulard P. DLBCL associated with chronic inflammation. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008: 245-246.
- Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells, and microorganisms. Nat Rev Cancer. 2013;13(11):759-771.
- Engels EA. Infectious agents as causes of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007 Mar;16(3):401-404.
- Ferreri AJM, Ernberg I, Copie-Bergman C. Infectious agents and lymphoma development: molecular and clinical aspects. J Internal Med. 2009;265(4): 421-438.
- Gilbert S, Affolter VK, Gross TL, Moore PF, Ihrke PJ. Clinical, morphological and immunohistochemical characterization of cutaneous lymphocytosis in 23 cats. Vet Dermatol. 2004;15(1): 3-12.
- Gilbert S, Affolter VK, Schmidt P, et al. Clonality studies of feline cutaneous lymphocytosis. Vet Dermatol. 2004; 15 (Suppl 1):
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. 2010;140(6): 883-899.
- Grivennikov SI, Karin M. Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev. 2010; 20(1):65-71.
- Gross TL, Ihrke PJ, Walder EJ, Affolter VK. Lymphocytic tumors. In: Skin Diseases of the Dog and Cat: Clinical and Histopathologic Diagnosis. 2nd Oxford, UK: Blackwell Science Ltd.; 2005:866-893.
- Jacobs RM, Messick JB, Valli VE. Tumors of the hemolymphatic system. In: Lymphoid tumors. 4th ed. Ames, IA, USA: Iowa State Press; 2002:119-198.
- Kidney BA. Role of inflammation/wound healing in feline oncogenensis: a commentary. J Feline Med Surg. 2008;10(2): 107-108.
- Louwerens M, London CA, Pedersen NC, Lyons LA: Feline lymphoma in the post-feline leukemia virus era. J Vet Intern Med. 2005;19: 329-335.
- Madewell BR, Gieger TL, Pesavento PA, Kent MS. Vaccine site-associated sarcoma and malignant lymphoma in cats: a report of six cases (1997-2002). J Am Anim Hosp Assoc. 2004;40(1): 47-50.
- Meichner K, von Bomhard W. Patient characteristics, histopathological findings and outcome in 97 cats with extranodal subcutaneous lymphoma (2007-2011). Vet Comp Oncol. 14 (S1), 8–20.
- Metgud RS, Doshi JJ, Gaurkhede S, Dongre R, Karle R: Extranodal NK/T-cell lymphoma, nasal type (angiocentric T-cell lymphoma): A review about the terminology. J Oral Maxillofac Pathol. 2011;15: 96-100.
- Roccabianca P, Avallone G, Rodriguez A, Crippa L, Lepri E, Giudice C, Caniatti M, Moore PF, Affolter VK. Cutaneous lymphoma at injection site: pathological, immunophenotypical, and molecular characterization in 17 cats. Vet Pathol. 2016;53(4):823-832.
- Savage KJ, Harris NL, Vose JM, Ullrich F, Jaffe ES, Connors JM, Rimsza L, Pileri SA, Chhanabhai M, Gascoyne RD, Armitage JO, Weisenburger DD. International peripheral T-cell lymphoma project. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. 2008;111: 5496-5504.
- Stein H, Warnke R, Chan W, Jaffe E, Chan J, Gatter K, Campo E. Diffuse large B-cell lymphoma, not otherwise specified. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008: 233-237.
- Suntz M, Failing K, Hecht W, Schwartz D, Reinacher M. High prevalence of non-productive FeLV infection in necropsied cats and significant association with pathological findings. Vet Immunol Immunopathol. 2010;136(1-2): 71-80.
- Valli VE, Jacobs RM, Norris A, et al. The histologic classification of 602 cases of feline lymphoproliferative disease using the National Cancer Institute working formulation. J Vet Diagn Invest. 2000;12(4): 295-306.
- Valli VEO, Kiupel M, Bienzle D, Wood DR. Hematopoietic System. In: Maxie MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. Vol 3. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016:103-267.
- Valli V. Veterinary Comparative Hematopathology. Ames, IA: Blackwell publishing; 2007.