Results
AFIP Wednesday Slide Conference - No. 22
1 March 2000
- Conference Moderator:
LTC Mark Martinez, Diplomate, ACVP
Pathology Division
U.S. Army Medical Research Institute of Infectious Disease
Ft. Detrick, Frederick, MD 21702-5011
- Return to WSC Case Menu
-
-
- Case I - 1023-97 (AFIP 2593958 )
-
- Signalment: Tissue from two adult female NCI Syrian
hamsters.
-
- History: One hamster was found recently dead while
one in another cage was hunched and trembling prior to euthanasia.
The second hamster had perineal fecal staining.
-
- Gross Pathology: (For one of the animals) Body weight
117.8 grams. The amount of body fat is considered normal. Minimal
postmortem autolysis is present. The glandular stomach and proximal
duodenum are diffusely reddened. Moderate numbers of ecchymoses
are present on the cecum, which is filled with green watery content.
The colon contains a small amount of viscous liquid content.
-
- Laboratory Results:
- Cultures of cecal content resulted in 2+ growth of E. coli
and failure to recover Citrobacter, Enterobacter, Klebsiella,
Morganella, Proteus, Providencia, Pseudomonas or Salmonella from
one animal. 1+ E. coli and Enterobacter were recovered from the
other. Anaerobic cultures were not available. Campylobacter plates
were negative.
-
- ELISA tests for Sendai virus, PVM, reovirus 3, LCM, Encephalitozoon
cuniculi, Tyzzer's bacillus and SV5 were negative. Clostridium
difficile toxin A antigen capture ELISA was strongly positive
for cecal contents of both hamsters.
-
- Contributor's Diagnosis and Comments: Widespread,
severe erosive enterocolitis, etiology Clostridium difficile.
This case was unusual in that there was no previous administration
of antimicrobial drugs. This factor was important to the occurrence
of the disease in rodents given clindamycin and other antibiotics.
Clostridium difficile is also a common nosocomial infection in
human beings, and has been reported in pigs, horses and dogs.
Disease is thought to be the result of toxin production by the
organism, and isolates that do not produce toxins are not pathogenic.
Toxin A causes fluid secretion and enterocyte damage when injected
into rodent intestine, while toxin B is not enterotoxic to animals,
but cytocidal in cell culture. Isolation of the organism is time-consuming
and sometimes inconsistent, so that antigen-capture ELISA to
detect toxins in gut contents is the most often used method of
confirming the diagnosis.
-
- AFIP Diagnoses:
- 1. Ileocecal junction: Enterocolitis, erosive, subacute,
diffuse, moderate, with crypt hyperplasia, submucosal edema,
and numerous luminal bacilli, Syrian hamster, rodent.
2. Lymph node, mesenteric: Lymphadenitis, subacute, diffuse,
moderate.
Conference Note: Clostridium difficile is a gram-positive
anaerobic bacillus, that produces its pathogenic effects through
two exotoxins, A (enterotoxin and proinflammatory agent) and
B (cytotoxin). Toxins bind to receptors on gut epithelial cells
resulting in inactivation of RhO cytoplasmic proteins, leading
to disaggregation of actin microfilaments and cell retraction.
Very young animals lack the epithelial receptors, and are thus
tolerant of very high levels of toxin. Clostridium difficile
has been associated with numerous disease syndromes in numerous
species: Antibiotic associated pseudomembranous colitis in numerous
species, colitis X and acute necrohemorrhagic enteritis in horses,
ulcerative colitis in dogs, and toxic megacolon and colonic perforation
in humans.
-
- The differential diagnosis discussed in conference also included
Campylobacter sp., Salmonella sp., E. coli, and Bacillus piliformis.
Gram stains performed at the AFIP demonstrate numerous, monomorphic,
Gram-positive bacilli.
-
- Contributor: Department of Veterinary Pathobiology,
University of Missouri, PO Box 6023, Columbia MO
-
- References:
- 1. Barlett JG: Clostridium difficile: History of its role
as an enteric pathogen and the current state of knowledge about
the organism. Clin Inf Dis 18:S265-S272, 1994
- 2. Brazier JS: Role of the laboratory in investigations of
Clostridium difficile diarrhea. Clin Inf Dis 16:S228-S233, 1993
- 3. Crawford JM: The gastrointestinal tract. In: Pathologic
Basis of Disease, eds. Cotran RS, Kumar V, Collins T, pp. 809-810.
WB Saunders, Philadelphia, PA, 1999
- 4. Kelly CP, Pothoulakis C, LaMont JT: Clostridium difficile
colitis. New Eng J. Med 330:257-262, 1994
- 5. Knoop FC, Owens M, Crocker IC: Clostridium difficile:
clinical disease and diagnosis. Clin Microb Rev 6:251-265, 1993
- 6. Liesenfeld O, Saeger F, Hahn H: Detection of Clostridium
difficile by enzyme immunoassay, tissue culture test and culture.
Infec 22:29-32, 1994
- 7. Lusk RH, Fekety R, Silva J, Browne RA, Ringler DH, Abrams
GD. Clindamycin-induced enterocolitis in hamsters. J. Infectious
Diseases 137:464-475, 1978
- 8. Wilson KH. The microecology of Clostridium difficile.
Clin Inf Dis 16:S214-S218, 1993
-
-
- Case II - 94263#10 ( AFIP 2694778)
-
- Signalment: 16-year-old, female, cynomolgus macaque
(Macaca fascicularis)
-
- History: This monkey was imported from Indonesia on
7/15/94. During the animal's quarantine period, it was noted
to have a draining cutaneous lesion on the cranial abdomen. Abdominal
palpation revealed firm, large masses subjacent to the draining
lesion. Euthanasia was elected approximately 6 weeks after importation
and a complete necropsy was performed.
-
- Gross Pathology: Presented for necropsy was a 3.8
kg, adult female monkey in good body condition with minimal post
mortem autolysis. There was a 0.6 cm focus of dried yellow material
3 cm caudal to the sternum on the left cranial abdomen.
The capsular surface of the spleen was covered by a large (6
X 5 X 2.7 cm) abscess which was adherent to the left abdominal
wall and communicated with the cutaneous draining lesion. On
section, the white fibrous capsule of the abscess was up to 2
mm thick and the abscess contained numerous small 2 mm round
to 2 X 1 cm elongate foci of yellow to greenish-yellow (purulent)
material. The splenic parenchyma contained many pinpoint (1 mm)
to coalescing (7 X 2 mm) abscesses. Lymphoid follicles were prominent
in other regions of the spleen.
-
- There was a 3 X 3 X 1.3 cm white firm mass on the capsule
of the right lateral lobe of the liver, which was adhered to
the body wall. The mass had a 2 mm thick fibrous capsule and
contained purulent material similar to that seen in the splenic
abscess. In the hepatic parenchyma subjacent to the abscess,
there was an irregular pale focus (2.5 X 2.0 X 4.0 cm) containing
pockets of purulent material. The left lateral lobe had a 2.5
X 2.0 X 1.0 cm abscess mainly involving the capsule of the liver
and adherent to the diaphragm. The abdominal surface of the left
diaphragm bore 2 raised white firm nodules (2.0 X 1.0 X 1.0 mm).
All abdominal lymph nodes were moderately enlarged and bulged
on cut surface.
-
- Laboratory Results:
- Clinical pathology:
|
Test |
Value |
|
|
WBC |
16,900 |
|
|
Segs |
56% |
|
|
Bands |
0% |
|
|
Monos |
1% |
|
|
Eos |
0% |
|
|
Basos |
0% |
|
|
Platelets |
Normal |
|
|
RBC |
4.8 |
|
|
Hgb |
7.9 |
|
|
MCV |
61 |
|
|
Hct |
29% |
|
|
BUN |
23 mg/dl |
|
|
TSP |
10.2 g/dl |
|
Moderate anisocytosis & poikilocytosis, slight polychromasia
- Microbiology results:
- Mycobacterial cultures of the spleen, liver and bone marrow
revealed no growth after 48 days of incubation. Routine cultures
initially interpreted as Pseudomonas aeruginosa, however, this
was later recharacterized as Burkholderia (Pseudomonas) pseudomallei.
-
- Virus status: Antibody-negative for SRV-1, SRV-2, SRV-5,
SIV and
STLV.
-
- Contributor's Diagnoses and Comments:
Gross Diagnoses:
1. Multifocal, chronic, hepatic abscesses with fibrous adhesion
2. Severe, chronic, multifocal to coalescing splenic abscess
with cutaneous fistulous tract.
3. Moderate abdominal lymphadenopathy.
-
- Histologic Diagnosis (this slide):
- Spleen: Severe, multifocal to coalescing, chronic, suppurative
splenitis with abscess formation
-
- Other Histologic Diagnoses (slides not submitted)
- 1. Liver: Hepatic capsular abscess with peritoneal adhesion
2. Liver: Severe subacute to chronic cholangiohepatitis with
biliary hyperplasia and fibrosis
3. Skin and body wall: Severe chronic suppurative dermatitis
and panniculitis with fibrosis (fistulous tract)
4. Lymph nodes (multiple intra-abdominal): Mild to moderate diffuse
follicular and paracortical lymphoid hyperplasia.
-
- Etiology :
Burkholderia pseudomallei, the causative agent of Melioidosis,
formerly known as Pseudomonas pseudomallei.
-
- We have seen melioidosis in 7 cynomolgus macaques imported
from Indonesia over a 5-year period. Of these animals, most developed
hepatic and/or splenic abscesses, some with draining skin lesions.
We have seen one case each of epididymitis and meningoencephalitis.
-
- This particular animal is one of three from this shipment
to have multiple abdominal abscesses (primarily splenic and hepatic).
The other two cases cultured out B. pseudomallei, while this
one initially cultured P. aeruginosa. Because of the history
of the other 2 animals, the culture was questioned. Further analysis
revealed Burkholderia pseudomallei.
-
- B. pseudomallei is a gram-negative, obligate anaerobe.
It is a soil and water saprophyte indigenous to Southeast Asia.
Melioidosis may present as septicemia, soft tissue abscesses,
pneumonitis, suppurative pneumonia, myocarditis, pericarditis,
lymphadenitis, infections of the male urogenital tract, and meningoencephalitis.
Many species have been affected by this disease, including man,
several species of nonhuman primates, horses, camels, goats,
cockatoos, and wallabies. Transmission to laboratory workers
has been documented.
AFIP Diagnosis:
- 1. Spleen and splenic capsule: Abscesses, multiple and coalescing,
chronic. cynomolgus macaque (Macaca fascicularis), non-human
primate.
2. Splenic capsule and pancreas: Adhesion, chronic, with inflammation,
pancreatic parenchymal atrophy, ductular hyperplasia, and regeneration.
Conference Note: Burkholderia pseudomallei (melioidosis)
has been referred to as the "remarkable imitator" because
of it's long clinical course and extremely variable clinical
signs. The organism also has the ability to emerge after remaining
latent in wild caught primates for up to 10 years. Recrudescence
has been attributed to environmental stress and/or compromise
of host defense mechanisms. The broad species range, zoonotic
potential and ability to spread insidiously through animal colonies
make this disease a significant problem. Frequently, the only
way to eradicate B. pseudomallei is through enzyme linked immunosorbent
assay testing for antibodies and euthanasia of all positive animals.
-
- Although most conference participants favored B. pseudomallei,
the following causes of multisystemic abscesses and splenitis
were also included in the differential diagnosis: Nocardia, Mycoplasma
sp., Corynebacterium pseudotuberculosis, Yersinia sp., Francisella
tularensis, Pseudomonas mallei, Listeria monocytogenes, Mycobacterium
and fungi.
-
- Contributor: Wake Forest University School of Medicine,
Department of Pathology, Section on Comparative Medicine, Medical
Center Boulevard, Winston-Salem, NC 27157-1040.
-
- References:
- 1. Ladds PW, Thomas AD, Pott B. Melioidosis with acute meningoencephalitis
in a horse. Aust Vet J 57:306-307, 1978
- 2. Schlech WF, Turchik JB, Westlake RE, Klein GC, Band JD,
Weaver RE: Laboratory-acquired infection with Pseudomonas pseudomallei
(Melioidosis). N Eng J Med 305:1133-1135, 1981
- 3. Thomas AD, Wilson AJ, Aubrey JN: Melioidosis in a sulphur-crested
cockatoo (Cacatua galerita). Aust Vet J 54:306-307, 1978
-
-
- Case III -970666 (AFIP 2596110)
-
- Signalment: Dog, German shepherd, female, 12-years-old
-
- History: This dog was presented at consultation with
anorexia, discordance, polypnea and weakness. Radiographs of
the thorax indicated the presence of a large volume of thoracic
fluid. A sample of this fluid was submitted for analysis to our
laboratory. An echography was performed and a thoracic mass located
cranial to the heart was suspected. After the diagnosis, the
dog was euthanatized at the owner's request.
-
- Gross Pathology: No complete necropsy was performed,
but opening of the pleural cavity showed numerous small nodules
distributed on all pleural surfaces within the thorax. Some nodules
were transmitted to our laboratory for histologic examination.
-
- Laboratory results: The pleural fluid was collected
twice. The first time, the thorax was drained of 1.3 liters of
pale, yellow, mildly opaque fluid. It had 14440 cells/ cubic
mm and 34 g/l of total proteins (estimated by refractometry).
The following day, the pleural fluid (only a few milliliters)
was serosanguineous and showed 29350 cells/ cubic mm and 29 g/l
of total proteins.
-
- Thoracic fluid cytology revealed numerous and irregular cellular
clusters associated with many inflammatory cells. The clusters
were composed of very irregular atypical vacuolated mesothelial
cells close together and which resemble carcinoma cells. The
cells present distinctive features of malignancy such a marked
variation in size, anisokaryosis, coarse chromatin pattern and
numerous prominent nucleoli. However, there were few mitotic
figures.
-
- Cytological examination of the second fluid presented the
same aspect associated with a highly hemorrhagic background.
-
- Contributor's Diagnosis and Comments: Malignant mesothelioma,
pleural cavity, dog.
- Light microscopic examination demonstrates within the fibrofatty
connective tissue of the pleura a papillary proliferation of
mesothelial cells. The papillary structures are supported by
fibrovascular stroma covered by one or several layers of cuboidal
or columnar neoplastic mesothelial cells. In some areas, there
is a sharp transition from normal to neoplastic tissue.
In several regions, nests and cords of tumor cells extend below
the surface and form a less organised proliferation, which is
associated with a fibrocellular stroma containing isolated spindle-shaped
cells or large anaplastic cells.
The neoplastic mesothelial cells have an eosinophilic cytoplasm
with distinct borders and often contain vacuoles. Nuclei have
multiple prominent nucleoli. There are marked anisokaryosis and
anisocytosis. Scattered mitotic figures are present. In all areas,
a large number of inflammatory cells are mixed with neoplastic
cells.
-
- Mesotheliomas are rare tumors arising from the mesothelium
lining the pleural, pericardial or peritoneal cavities. Cases
of involvement of the tunica vaginalis of the testis have been
reported also. In domestic animals, two distribution patterns
can be observed: cattle and sheep develop tumors in fetal, newborn
and young animals whereas in others species, adult or aged animals
are affected. In dogs, the pleura is the main site of development
after the pericardium and the peritoneal cavity. The clinical
sign associated with pleural mesothelioma is essentially dyspnea
caused by accumulation of fluid in the pleural cavity. Collapse
of lung lobes can be a consequence of the effusion also. Analysis
of the effusion fluid usually shows a milky or bloody color,
low protein content, may show free erythrocytes and a moderate
number of nucleated cells. These latter cells are sometimes only
leukocytes but tumor cells can be found also.
-
- On gross examination, tumors are formed either by small nodules,
sessile or pedunculated, from a few millimeters to 10 centimeters
in diameter or by villous projections arising from the serosal
surface. Depending of the amount of hemorrhage, the color varies
from gray-white to red. Some fibrous or sclerosing forms have
been reported more rarely.
-
- Mesotheliomas present three main histological patterns: a
form resembling fibrosarcoma with spindle-shape cells, a sclerosing
pattern and, the most common, an epithelioid type. In this latter
form, cuboidal epithelioid cells cover papillary projections
made of spindle-shape cells and conjunctivo-vascular stroma.
Sometimes cells can form tubules or rosettes and mitoses are
usually not numerous. Sclerosing mesotheliomas are composed of
large anaplastic cells with sometimes multinucleated giant cells.
On histologic examination, mesotheliomas can be either benign
or malignant. The malignant type is more common and frequently
leads to local extension into the coelomic cavities. Involvement
of local lymph nodes can occur, but hematogenous metastatic dissemination
is extremely rare. Concerning the epithelioid pattern differential
diagnosis must be made with metastasis from an adenocarcinoma
located in another site, mammary gland or ovary for example.
Lipomas, liposarcomas and more rare tumors such as ganglioneuromas
can also develop from the serous membranes.
-
- In humans, an association between malignant mesothelioma
and asbestos exposure has been well established. In domestic
animals, however, few tumors have been examined for asbestos
fibers but there is little evidence for a relationship between
asbestos inhalation and mesothelioma. Concerning newborn cattle,
a congenital origin has been found for mesotheliomas.
-
- AFIP Diagnosis: Malignant mesothelioma, German shepherd
dog, canine.
-
- Conference Note: Mesotheliomas occur with the greatest
frequency in cattle and dogs, but have been reported in most
domestic species. In cattle, they occur most often as a congenital
neoplasm in fetuses or young calves. Mesothelioma arising from
the tunica vaginalis is one of the most common tumors of the
male Fischer 344 rat.
-
- The tumors may cause the accumulation of large amounts of
fluid, resulting in ascites, cardiac insufficiency, cardiac tamponade,
or respiratory distress depending upon the location of the tumor.
Adhesions are often formed between the affected serosal surface
and adjacent organs.
-
- Ultrastructurally, neoplastic mesothelial cells have a prominent
basal lamina, well-developed microvilli, desmosomes, abundant
rough endoplasmic reticulum, and mitochondria.
-
- The mucicarmine stain with and without hyaluronidase can
help distinguish mesothelioma from adenocarcinoma. The presence
of mucicarminophilic, hyaluronidase resistant material within
cytoplasmic vacuoles supports adenocarcinoma. By immunohistochemistry,
neoplastic cells of mesothelioma are typically positive for both
keratin and vimentin. Carcinomas are usually keratin positive
and vimentin negative.
-
- This neoplasm did not stain with mucicarmine. Immunohistochemically,
the neoplastic cells are diffusely positive for keratin and multifocally
positive for vimentin. Thus, histomorphology and immunohistochemistry
support mesothelioma.
-
- Contributor: Ecole Vétérinaire dAlfort,
Laboratoire dAnatomie Pathologique, 7, Avenue du Général
de Gaulle, 94704 Maisons Alfort - France.
-
- References:
- 1. Barker IK: The peritoneum and retroperitoneum in Pathology
of Domestic Animals, vol. 2, eds. Jubb, KVF, Kennedy, PC, Palmer,
4th ed., pp. 31-35. Academic Press, Inc., San Diego, CA, 1993
- 2. Forbes DC, Matthews BR: Abdominal mesothelioma in a dog.
Can Vet J 32:176-177, 1991
- 3. Foumel C, Magnol JP, Guelfi JF: Color atlas of cancer
cytology of the dog and cat, PMCAC Ed, pp.82-83, 1994
- 4. Harbison ML, Godleski JJ: Malignant mesothelioma in urban
dogs. Vet. Path., 20:531-540,1983.
- 5. Head KW: Tumors of the alimentary tract. In: Moulton JE:
Tumors in domestic animals, 3rd ed., pp. 422-427. University
of California Press, San Diego, CA, 1990
- 6. McDonough SP, MacLachlan NJ, Tobias AH: Canine pericardial
mesothelioma. Vet Path 29:256-260, 1992
- 7. Smith DA, Hill FWG: Metastatic malignant mesothelioma
in a dog.
J Comp Pathol 100:98-101,1989
-
-
- Case IV - G5594-99 (AFIP2715642)
-
- Signalment: Adult, male, golden-headed lion tamarin
(Leontopithecus chrysomelas)
-
- History: This animal was from a group of 11 animals
separately housed in a zoo. All animals showed clinical symptoms
of conjunctivitis, blepharitis, and ulceration of muco-cutaneous
junctions of the lips. Eight animals died, three recovered after
severe illness. Antibodies against measles virus and herpes simplex
virus (HSV 1,2) were negative in one tested animal. One of the
dead animals was submitted to the German Primate Centre for diagnostic
purposes.
-
- Gross Pathology: The animal showed severe ulcerative
stomatitis and inflammation of muco-cutaneous junctions of the
lips. Furthermore an erosive to ulcerative conjunctivitis, esophagitis
and gastritis were found. Within the liver there were multiple
foci of necrosis.
-
- Laboratory Results:
Staphylococcus aureus, Streptococcus pneumoniae viridans-group
and Streptococcus bovis II D were cultured from the mucous
membranes of the eyes and oropharynx.
Contributor's Diagnoses and Comments:
- 1. Hepatitis, necrotizing, acute to subacute, multifocal,
moderate, histiocytic with few lymphocytes and granulocytes with
hemorrhage and hepatocytic amphophilic to eosinophilic intranuclear
inclusions with and without halo.
2. Glossitis, ulcerative, subacute, multifocal moderate to severe,
granulocytic and histiolymphocytic, with ballooning degeneration
of epithelial cells, multinucleate giant cells with amphophilic
to eosinophilic intranuclear inclusions, focal necrotizing vasculitis
in the muscle and superficial bacterial growth.
3. Conjunctivitis/Cheilitis, ulcerative, acute to subacute, focal,
moderate, granulocytic and histiolymphocytic, with ballooning
degeneration, spongiotic vesicles, multinucleate giant cells,
amphophilic to eosinophilic intranuclear inclusions in epithelial
cells, focal sebaceous adenitis, mineralization and superficial
bacterial growth.
-
- Electron microscopy of liver tissue revealed numerous viral
particles with nucleocapsid and characteristic symmetry within
nuclei of hepatocytes in the periphery of the necrotic foci.
The viral particles measured approximately 100 nm in diameter.
Occasionally virions, 150 to 200 nm in diameter, obtaining an
envelope from the inner membrane of the nucleus could be seen.
Histologic and electron microscopic findings are consistent with
alphaherpesvirus infection (Herpes simplex or Herpes
tamarinus).
-
- Erosive to ulcerative stomatitis with multinucleated giant
cells containing intranuclear inclusions in connection with necrotizing
hepatitis, where inclusion bodies can be seen also, are characteristic
for an alphaherpesvirus infection induced by human herpesvirus
1,2 (H. simplex) or saimirine herpesvirus 1 (Herpesvirus tamarinus
syn. H. platyrrhinae). Both viruses lead to systemic infection
with identical gross and microscopic lesions. Furthermore, they
can not be distinguished by electron microscopy.
-
- Several species of marmosets and tamarins are susceptible
to certain herpesviruses asymptomatically carried by squirrel
monkeys, macaques and humans. In the case of H. simplex humans
are the natural host or reservoir, and infected people like zoo
visitors or technicians can excrete the virus in the absence
of visible lesions. H. tamarinus is carried by squirrel
monkeys (Saimiri sciureus), in which clinical disease is rarely
reported. Because of the detection of neutralizing antibodies,
cinnamon ring-tailed monkeys (Cebus albifrons) and spider
monkeys (Ateles spp.) are considered to be natural reservoir
hosts, too. Infection of marmosets, tamarins, owl monkeys and
tree shrews induces sporadic episodes of generalized fatal infection
with high morbidity and mortality eg. in zoos.
-
- In this case anamnestical investigations showed that the
group of golden-headed lion tamarins could have had contact with
zoo visitors or transmission of H. tamarinus from other
cebids could have occurred via zoo technicians. A retrospective
diagnostic differentiation of both viruses can only be carried
out by PCR techniques in necropsy material or by antibody analysis
of the surviving animals.
-
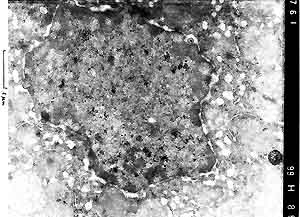
- Case 22-4. Liver (per contributor) TEM. Scattered
within the nucleus are multiple, 100nm diameter, viral particles
(central nucleoid surrounded by a capsid = [nucleocapsid]). Occasional
virions, 150-200nm in diameter, are acquiring an envelope from
the inner nuclear membrane.
-
- AFIP Diagnoses:
- 1. Liver: Hepatitis, necrotizing, acute to subacute, subcapsular,
multifocal, moderate, with syncytia and eosinophilic intranuclear
inclusion bodies, golden headed tamarin (Leontopithecus chrysomelas),
nonhuman primate.
2. Lip and eyelid: Inflammation, necrotizing, acute, multifocal,
moderate to severe, with ulceration, syncytia and eosinophilic
intranuclear inclusion bodies.
Conference Note: A number of alpha-herpesviruses naturally
infect nonhuman primates, including Herpes T of squirrel monkeys,
H. simiae (B virus) of macaques, SA-8 of African green
monkeys and simian varicella. Human alpha-herpesviruses are HSV-1,
HSV-2 and H. varicella/H. zoster. Herpes simplex infection
in marmosets, tamarins and owl monkeys cannot be differentiated
clinically, grossly or histologically from herpesvirus T infection.
Specific immunohistochemical staining, virus isolation or specific
molecular techniques are necessary for precise identification
of the causative virus. When infected with HSV or Herpes T, marmosets,
tamarins and owl monkeys may develop a generalized disease, often
with encephalitis and death. Accordingly, all macaques should
be handled with appropriate caution, species of monkeys should
not be mixed, and persons with active HSV infections should be
precluded from contact with susceptible nonhuman species.
-
- Contributor: German Primate Centre, Department of
Veterinary Medicine and Primate Husbandry, Kellnerweg 4, 37077
Göttingen, Germany.
-
- References:
1. Bruno SF, Liebhold MM, Mätz-Rensing K, Romao MAP, Didier
A, Brandes F, Bressan ACS, Kaup FJ: Herpesvirus-Infektion bei
freilebenden Schwarzpinseläffchen (Callithrix penicillata,
E. Geoffroyi 1812) im Parque Estadual da Serra Tiririca Niterói,
Rio de Janeiro, Brasilien. BMTW 110:427-430, 1997.
2. Hunt RD: Herpes simplex Infection. In: Nonhuman Primates I,
eds. Jones TC, Mohr U, Hunt RD, pp. 82-86. Springer-Verlag, Berlin,
New York, 1993
- 3. Hunt RD, Blake BJ: Herpes platyrrhinae Infection. In:
Nonhuman Primates I, eds. Jones TC, Mohr U, Hunt RD, pp. 100-103.
Springer-Verlag, Berlin, New York, 1993
- 4. Juan-Sallés C, Ramos-Vara JA, Prats N, Solé-Nicolás
J, Segalés J, Marco AJ: Spontaneous herpes simplex virus
infection in common marmosets (Callithrix jacchus). J Vet Diagn
Invest 9:341-345, 1997
- 5. Mansfield K, King N: Viral Diseases. In: Nonhuman Primates
in Biomedical Research: Diseases, eds. Bennett BT, Abee CR, Hendrickson
R, pp. 5-12. Academic Press, San Diego, New York, 1998
-
-
- J Scot Estep, DVM
Captain, United States Army
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: estep@afip.osd.mil
-
- * The American Veterinary Medical Association and the American
College of Veterinary Pathologists are co-sponsors of the Registry
of Veterinary Pathology. The C.L. Davis Foundation also provides
substantial support for the Registry.
- Return to WSC Case Menu
