Results
AFIP Wednesday Slide Conference - No. 21
Febuary 23, 2000
- Conference Moderator:
Dr. Peter C. Kennedy, Diplomate, ACVP
University of California at Davis
College of Veterinary Medicine
Davis, California 95616
-
- Return to WSC Case Menu
-
- Case I - 17145-99 (AFIP 2687067)
-
- Signalment: Canine, schnauzer, female, twelve-year-old.
-
- History: This dog presented with a markedly distended
abdomen. A laparotomy was performed. The abdomen was filled
with clear amber fluid. The right ovary was enlarged and measured
approximately 3.5 - 4 cm in diameter and an ovariohysterectomy
was performed. A section of the ovary was submitted for histopathologic
examination. Six months following surgical removal, the dog
remains clinically normal.
-
- Contributor's Diagnosis and Comments: Ovarian sex-cord
stromal cell tumor (luteoma)
-
- These sections of ovarian mass contain dense sheets and nests
of round to polyhedral cells with abundant, finely vesiculated
eosinophilic cytoplasm. There are broad bands of fibrovascular
stroma which separate areas of the mass. In some areas, the
mass is disrupted by hemorrhage and edema. Some sections contain
a reactive layer of mesothelial cells which are arranged in small
papillary projections from the serosal surface. Remnants of
normal ovary are present in some sections.
-
- Sex-cord stromal cell tumors, in order of decreasing frequency,
are granulosa cell tumor, thecoma, and luteoma. Three distinguishing
types of granulosa cell tumors are those that contain Call-Exner
bodies, cells which are tall and palisade along basement membranes
(Sertoli cell-like), and those which are arranged in a poorly
differentiated sarcomatous pattern. Thecoma is a rare tumor
found in cows and consists of spindle or star-shaped cells.
Luteomas are rare tumors in domestic animals and are generally
considered to be benign. Origin of luteal cells in the ovary
is theca interna and stratum granulosum.
-
- AFIP Diagnosis: Ovarian interstitial cell tumor (luteoma),
schnauzer, canine.
-
- Conference Note: Ovarian tumors are uncommon in all
domestic species. There are three categories of primary ovarian
tumors: epithelial (papillary adenoma and cystadenoma, papillary
adenocarcinoma, and rete adenoma), germ cell (dysgerminoma, teratoma,
and embryonal carcinoma) and sex-cord stromal (granulosa cell
tumor, thecoma, and interstitial cell tumor (luteoma, lipid cell
tumor, steroid cell tumor)).
-
- Sex cord-stromal tumors (most often granulosa cell tumors)
are most common in the mare, cow and bitch. They are the most
frequent primary ovarian tumor in all domestic animal species,
with the possible exception of the dog, which have about equal
occurrence of sex cord-stromal tumors and papillary cystadenocarcinomas.
In mares, about 80% of ovarian tumors are sex cord-stromal tumors,
specifically granulosa cell tumors. Sex cord-stromal tumors
are usually unilateral and benign. Although they may occur in
young animals, the incidence increases with age. Malignant sex
cord-stromal tumors occur most often in cats, less often in dogs
and cattle, and rarely in horses. Sex cord-stromal tumors have
also been reported in rats, mice, rhesus and squirrel monkeys,
domestic fowl, ferrets and pet birds. Sex cord-stromal tumors
account for approximately 5% of ovarian neoplasms in humans,
with approximately two-thirds occurring in postmenopausal women.
-
- The cell of origin of interstitial cell tumors has not been
clearly identified, and may vary between species and between
tumors within a species. The most distinctive histomorphological
feature is the abundant eosinophilic cytoplasm that contains
numerous lipid-type steroid vacuoles. Most of the reported interstitial
cell tumors have been hormonally active. Hyperadrenocorticism
has been associated with interstitial cell tumors in humans and
in one dog.
-
- Contributor: Veterinary Diagnostic Center, Fair Street
and East Campus Loop, Lincoln, NE, 68583-0907
-
- References:
- 1. Jones TC, Hunt RD, King NW: Veterinary Pathology, 6th
ed., pp. 1159-1162. Williams & Wilkins, Philadelphia, PA,
1997
- 2. Kennedy PC, Cullen JM, Edwards JF, Goldschmidt MH, Larsen
S, Munson L, Nielsen S: Histological Classification of the Tumors
of the Genital System of Domestic Animals. In: World Health
Organization, Histological Classification to Tumors of Domestic
Animals, ed. Schulman FY, 2nd ed., vol. 4, pp. 24-28. The Armed
Forces Institute of Pathology, Washington, DC, 1998
- 3. Nielsen SW, Kennedy PC: Tumors of the Genital Systems.
In: Tumors in Domestic Animals, ed. Moulton JE, 3rd ed., pp.
503-507, University of California Press, 1990
- 4. Yamini B, VanDenBrink PL, Refsal KR: Ovarian steroid
cell tumor resembling luteoma associated with hyperadrenocorticism
(Cushing's disease) in a dog. Vet Pathol 34:57-60, 1997
-
-
- Case II - NADC BK13 (AFIP 2679736)
-
- Signalment: 20-year-old, Morgan-cross quarter horse,
gelding, equine.
-
- History: The horse was donated to the Oregon State
University's Veterinary Teaching Hospital, Corvallis, OR. Upon
presentation, the gelding was in poor body condition and demonstrated
severe hind leg ataxia. Body temperature, heart and respiration
rates were within normal limits. A clinical diagnosis of equine
protozoal myeloencephalitis (EPM) was made. The gelding was
injected intramuscularly with 0.2mg/kg dexamethasone s.i.d. for
12 days prior to euthanasia in an attempt to increase the parasite
burden.
-
- Gross Pathology: No grossly evident lesions were present.
-
- Laboratory results: Cerebrospinal fluid was positive
for Sarcocystis neurona antibodies by western blot examinations
as described by Granstrom et al. (J Vet Diagn Invest 1993. 5:88-90)
-
- Contributor's Diagnosis and Comments: Spinal cord
(thoracic): Myelitis, lymphohistiocytic, mild, multifocal with
protozoan parasites.
-
- Etiology: Neospora caninum
-
- Histologic lesions attributed to protozoal infection were
confined to the cerebrum and thoracic spinal cord. The cerebral
lesions consisted of multifocal, perivascular, variably-sized
granulomas composed of large numbers of epithelioid macrophages
containing few protozoa. In the thoracic spinal cord (submitted
tissue), there are multifocal areas in the ventral and lateral
peripheral white matter with mild to moderate infiltrates of
macrophages and fewer lymphocytes around small blood vessels.
Variably-sized, non-encysted groups (5 to 50 mm in diameter)
of protozoa are often present adjacent to inflammatory foci.
The degree of inflammation and numbers of protozoa vary between
sections. In some sections containing few groups of protozoa,
there is little or no evidence of inflammation.
-
- The protozoa stained by immunohistochemistry with both polyclonal
and monoclonal antibodies to Neospora caninum, but not
with antibodies to Sarcocystis neurona or Toxoplasma
gondii. In addition, ultrastructural features consistent
with N. caninum including electron-dense rhoptries were
observed. However, a recent report (Marsh et al. 1998) describing
a new Neospora species isolated from a horse spinal cord indicates
that without close examination of differences in immunoreactive
proteins and nucleotides comprising the internal transcribed
spacer I region, N. caninum and N. hughesi cannot
be distinguished.
-
- It is uncertain at present whether N. caninum or N. hughesi
are frequent causative agents of EPM. In the majority of cases
of EPM, S. neurona is presumed to be the causative agent. In
the present case, the CSF of the horse was positive for S. neurona
antibodies by western blot. The etiologic diagnosis was based
on the demonstration of Neospora in tissues, as the presence
of specific antibodies does not necessarily indicate active disease.
-
- Unusual features in this case, ascribed to the corticosteroid
administration, include minimal inflammation, large numbers of
intralesional tachyzoites, lack of tissue cysts, and lesions
confined to peripheral areas suggesting intrathecal spread of
the infection.
-
- AFIP Diagnosis: Spinal cord: Myelitis, nonsuppurative,
submeningeal, multifocal, mild to moderate, with multifocal axonal
degeneration, myelin sheath swelling, mild meningitis, and protozoal
tachyzoites.
-
- Conference Note: Neospora caninum (Phylum Apicomplexa,
Family Sarcocystidae) is a protozoan that infects wild and domestic
canids (definitive hosts), and ruminants and horses (intermediate
hosts). Although Neospora has only recently been associated
with equine protozoal myeloencephalitis, it has been proposed
as a possibly significant cause of EPM previously misdiagnosed
as Sarcocystis neurona. As demonstrated in this case, positive
cerebrospinal fluid titers for S. neurona do not confirm active
infection. Ultrastructurally, Neospora sp. has many electron-dense
rhoptries and may be found within a parasitophorous vacuole.
Merozoites of Sarcocystis sp. lack rhoptries and meronts are
directly in the host cell cytoplasm. Toxoplasma gondii has few,
variably electron-dense rhoptries and is found within a parasitophorous
vacuole. Immunohistochemistry, PCR, and/or electron microscopy
are necessary for definitive diagnosis.
-
- Contributor: USDA, ARS, National Animal Disease Center,
2300 Dayton Avenue, P0 Box 70, Ames, IA 50010
-
- References:
- 1. Granstrom DE, Dubey JP, Davis SW, Fayer R, Fox JC, Poonacha
KB, Giles RC, Corner PF: Equine protozoal myeloencephalitis:
antigen analysis of cultured Sarcocystis neurona merozoites.
Vet Diagn Invest 5:88-90, 1993
- 2. Hamir AN, Tornquist SJ, Gerros TC, Topper MJ, Dubey JP:
Neospora caninum-associated equine protozoal myeloencephalitis.
Vet Parasitol 79:269-274, 1998
- 3. Marsh AE, Barr BC, Packham AE, Conrad PA: Description
of a new Neospora species (Protozoa: Apicomplexa: Sarcocystidae).
J Parasitol 84(5):983-991, 1998
- 4. McAllister MM, Dubey JP, Lindsay DS, Jolley WR, Wills
RA, McGuire AM: Dogs are definitive hosts of Neospora caninum
(Rapid Communication). Int J Parasitol 28:1473-1478, 1998
-
-
- Case III - 97-1130 (AFIP 2595291)
-
- Signalment: 8.5 month gestation crossbred Angus fetus.
-
- History: This bovine fetus was 1 of 2 fetuses that
were found dead the same day from a group of heifers in a university
herd. Vaccinations against the major abortigenic agents of cattle
were current, and husbandry was described as good to excellent.
Placenta was presented with the fetus.
-
- Gross Pathology: The animal examined was an approximately
30 kg fetal calf. Necropsy yielded minimal to mild autolysis
of the fetus. There were no gross lesions in the fetus or placenta.
The lungs were inflated, consistent with the delivery of a live
calf.
-
- Laboratory Results:
- Bacteriological and mycological procedures yielded only bacterial
contaminants from cultures of the placenta, liver, and lung.
Virus isolation techniques, performed on spleen, kidney, liver,
and lung were negative for all viral agents. Fluorescent antibody
techniques were negative for the IBR and BVD viruses and negative
for Leptospira species.
-
- Histopathology was performed on 6 mm sections of multiple
tissues. In sections of placenta, there was acute to subacute
necrotizing placentitis. Necrotic debris at the chorionic villi
was admixed with intact and degenerate neutrophils and extracellular
protozoal tissue cysts. The cysts were ovoid, measuring 100
x 150 mm, with cyst walls less than 1 mm in thickness. These
tissue cysts contained 20-50 basophilic, pyriform to spindled
merozoites that were 2-3 mm long. Occasional merozoites formed
rosettes that appeared to be within the vascular endothelium.
There was focally extensive necrosis of trophoblastic epithelium,
most prominent at the tips of the chorionic villi. In the subtrophoblastic
stroma, there were small numbers of disseminated neutrophils
and mononuclear cells, with multifocal protozoal cysts that were
usually located within the cytoplasm of rounded and markedly
hypertrophic endothelial cells of the placental stromal blood
vessels. There was multifocal necrotizing vasculitis within
occasional affected vessels. Endothelial cells were pyknotic
and were admixed with neutrophils, cellular debris, and occasional
protozoal cysts.
-
- Sections from various other tissues examined included brain,
skeletal muscle, myocardium, thymus, liver, lung, and kidney.
Protozoal cysts, similar to those located within the placenta,
were also located in small to large numbers within arterial and
capillary endothelial cells of all organs examined. Focal zones
of necrosis were associated with the protozoal cysts in the affected
organs.
-
- Contributor's Diagnoses and Comments:
- 1. Placenta: Placentitis, moderate, multifocal, acute to
subacute, necrotizing, with intraendothelial and extracellular
protozoal meronts, etiology most consistent with Sarcocystis
cruzi (syn., Sarcocystis bovicanis) or other Sarcocystis
sp.
2. Placenta; blood vessels: Vasculitis, moderate, multifocal,
acute to subacute, necrotizing, with intraendothelial protozoal
meronts.
-
- Sarcocystis species are two-host protozoal coccidian parasites
of the Phylum Apicomplexa that are considered to be host-specific.
The group is characterized by the presence of resistant spore
stages and by the production of sexual and asexual stages of
the life cycle. Sarcocystis species are frequently named after
the intermediate and definitive hosts (eg-S. bovicanis).
Numerous species of Sarcocystis have been identified, and most
vertebrates appear to be either intermediate hosts or definitive
hosts or both for several of these agents. As a general rule,
the intermediate hosts are herbivores, with carnivores being
the definitive hosts.
-
- The life cycles of many species have been defined. In the
case of S. cruzi, oocysts sporulate in the intestine of the dog
to form sporocysts that pass with the feces into the external
environment. The sporocysts are ingested by cattle and excyst
as sporozoites in the intestine. From the bovine intestinal
lumen, the sporozoites migrate to arterial vessels and develop
into first generation meronts in the endothelial cells. Mature
first generation merozoites from the meronts emerge and develop
into secondary meronts within capillary endothelial cells. The
liberated second generation merozoites emerge and enter mononuclear
cells. These merozoites leave the circulation to enter the myofibers
of myocardium or skeletal muscle or occasionally neurons to form
immature sarcocysts that are not yet infective. The sarcocysts
form metrozoites that multiply and eventually develop into bradyzoites,
which are the intramuscular stages infectious to carnivores.
The life cycle is continued by ingestion of the infected muscle
tissue by the definitive carnivore host. Digestion of the sarcocysts
in the intestine of the dog liberates tachyzoites that invade
the intestinal epithelium and develop directly into macro- and
microgametocytes. Gametogony (fertilization) then results in
the formation of unsporulated oocysts that sporulate and are
then released to the environment as sporocysts.
-
- Although mature sarcocysts that contain bradyzoites are extremely
common in the skeletal and myocardial myofibers of cattle, the
organisms are seldom identified in the tissues of aborted bovine
fetuses in any of the tissue forms. When they occur in the fetus
or term calf, the lesions consist of acute to subacute inflammation
of brain, hepatic, renal, and other tissues. The large number
of tissue cysts that were seen in the placenta and other tissues
of this case was considered highly unusual. Diagnosticians at
Kansas State University considered that the dam in this case
could have been either immunologically naive to Sarcocystis at
the time of infection, or may have been immune-compromised by
weather, shipping, or the stress of late pregnancy. A detailed
history of this individual heifer was unavailable.
-
- The production of occasional rosettes by merozoites in the
immature meronts seen in these placental tissues was considered
diagnostic for Sarcocystis species. The life cycle of Sarcocystis
species is 90 or more days. If immature sarcocysts are present
in the intermediate host myofibers, it is assumed that the infection
is of a duration of 60 days or longer. As mature sarcocysts
were not identified in the fetal or placental tissues of this
case, it was assumed that the infection of the dam had occurred
less than 60 days previously. Most abortions due to S. cruzi
in cattle occur late in gestation, and the calves often live
for a short time after parturition, as in this case.
-
- Other protozoal agents that should be considered in the differential
diagnosis in late gestation bovine abortions include Neospora
sp. and Toxoplasma. Although extremely common as an infectious
cause of bovine abortion, Neospora sp. tissue cysts have been
reported only in the brain of aborted fetuses, with tachyzoites
located in myocardial and skeletal muscle, liver, and brain.
Toxoplasma are not thought to be associated with naturally occurring
abortions in cattle, but are common agents of abortions in sheep
and lesions are usually more necrotizing than Sarcocystis sp.
infections.
-
- Further diagnostic tests that could be considered in cases
of protozoal abortions would include immunohistochemical testing
to identify zoites or tissue cysts of Sarcocystis sp., Toxoplasma,
and Neospora sp. Additionally, electron microscopy may be used
to differentiate these morphologically similar organisms. Paired
serological testing of the serum of the dam has been used to
diagnose S. cruzi abortions. Fetal serology for Sarcocystis
sp. is unrewarding.
-
- AFIP Diagnosis: Placenta: Placentitis, necrotizing,
subacute, diffuse, moderate, with necrotizing vasculitis, and
numerous protozoa, etiology consistent with Sarcocystis sp.,
Angus cross-bred fetus, bovine.
-
- Conference Note: The contributor has provided an excellent
summary of this entity.
-
- Contributor: Veterinary Diagnostic Laboratory, Kansas
State University, 1800 Denison Avenue, Manhattan, KS 66506-5601
-
- References:
- 1. Anderson ML, Barr BC, Conrad PA: Protozoal causes of
reproductive failure in domestic ruminants. Vet Clin NA: Food
Ani Pract 10:449-451, 1994
- 2. Gardiner CH, Fayer R, Dubey JP: An Atlas of Protozoan
Parasites in Animal Tissues. 2nd ed., pp. 41-46, Armed Forces
Institute of Pathology, Washington, DC, 1998
- 3. Kennedy PC, Miller RB: The female genital system. In:
Pathology of Domestic Animals, ed. Jubb KVF, Kennedy PC, Palmer
N, 4th ed., vol.3, pp. 425-426. Academic Press, San Diego, CA,
1993
- 4. Stalheim OH: Bovine abortion caused by Sarcocystis. In:
Laboratory diagnosis of livestock abortion, ed. Kirkbride CA,
3rd ed., pp. 153-155. Iowa State University Press, Ames, IA,
1990
- 5. Wouda W, Moen AR, Visser IJR: Bovine fetal neosporosis:
a comparison of epizootic and sporadic abortion cases and different
age classes with regard to lesion severity and immunohistochemical
identification of organisms in brain, heart, and liver. J Vet
Diagn Invest 9:180-185, 1997
-
-
- Case IV - Y-5363 (AFIP 2688416)
-
- Signalment: 13-year-old, female, domestic longhair
feline, Felis catus
-
- History: An ovariohysterectomy was performed on the
cat for treatment of pyometra. The uterus and ovaries were submitted
for histopathology. No additional history was provided.
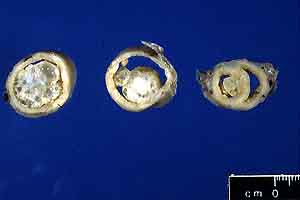
- Case 21-4. Uterus. Extending from the uterine mucosa
into the lumen is a multicystic white-tan mass.
-
- Gross Pathology: The formalin-fixed uterus was moderately
distended and filled with opaque brown fluid. The uterine horns
contained six broad-based to pedunculated endometrial nodules
ranging from 0.5 cm to 2 cm in diameter (gross photo of cross-sectioned,
formalin-fixed uterus). Nodules were located throughout the
uterine horns from near the uterine bifurcation (section on the
right) to the tips of the horns (section on the left).
-
- Contributor's Diagnosis and Comments: Cystic endometrial
hyperplasia with hyperplastic endometrial polyps, chronic suppurative
endometritis, and pyometra with Gram-negative bacilli.
-
- Microscopically, the uterus is characterized by diffuse cystic
endometrial hyperplasia with multifocal pedunculated masses of
cystic endometrial glands supported by well-vascularized connective
tissue stroma. The stroma varies from loose and edematous to
dense and collagenous; in some of the masses, smooth muscle is
a prominent component of the stroma. The endometrial lamina
propria contains numerous plasma cells, lymphocytes, neutrophils,
and focal accumulations of hemosiderin-laden macrophages. Purulent
exudate in the endometrial glands and lumen of the uterus contains
numerous Gram-negative bacilli. Both ovaries from this cat contained
multiple follicles and no corpora lutea.
-
- Among domestic species, endometrial hyperplasia (EH) may
occur following prolonged estrogen or progesterone influence
on the endometrium. Unlike EH in women, EH in domestic animals
is not considered a precancerous lesion. Two main events influence
the development of endometrial hyperplasia (EH) in dogs and cats.
First, the endometrial epithelium is stimulated by estrogen,
to produce receptors for progesterone. Second, progesterone from
corpora lutea (CL) stimulates growth of endometrial epithelium.
Cats are induced-ovulators, but CL in queens may also occur
spontaneously. In dogs and cats, hyperplastic endometrial polyps
are thought to arise from focal areas of cystic EH. In one study,
EH in cats was commonly found in queens 5 years-of-age and older
and was not associated with CL; therefore, EH in cats may be
due to prolonged estrogenic stimulation. In this same study,
there was a positive correlation between endometritis/pyometra
and CL. In general, only half of queens with pyometra at surgery
or death have CL, and the relationship between EH and pyometra
in cats is unclear.
-
- AFIP Diagnosis: Uterus: Cystic endometrial hyperplasia
with endometrial polyp and chronic suppurative endometritis.
-
- Conference Note: Cystic endometrial hyperplasia (CEH)
in the bitch is associated with increased progesterone from retained
corpora lutea following estrogen priming of the endometrium.
In women, cows, mares, and ewes, endometrial hyperplasia is
associated only with estrogen stimulation, due to cystic follicles,
estrogenic plants, or granulosa cell tumors. Investigations
of uterine disease in cats by Potter et al, found that retained
CL were associated with pyometra and endometritis, but not with
endometrial hyperplasia, suggesting that progesterone is not
required in the pathogenesis of feline CEH. In a recent study
by Perez et al that compared feral cats to colony-reared cats,
feral cats had 3 times more ovarian interstitial cells, lower
serum estradiol levels and zero incidence of CEH, while domestic
cats had an 88% incidence of CEH in cats over 5 years of age
and 30% incidence in 2-4 year-old cats. In the cat, ovarian
interstitial cells have a histologic appearance that suggests
steroid production, and are believed to arise from the theca
interna of atretic follicles, but what steroids they secrete
remains to be determined. The pathogenesis of feline CEH may
be multifactorial and requires further study.
-
- Contributor: Diagnostic Laboratory Service, College
of Veterinary Medicine, Mississippi State University, Mississippi
State, MS 39762
-
- References:
- 1. Gelberg HB, McEntee K: Hyperplastic endometrial polyps
in the dog and cat. Vet Pathol 21:570-573, 1984
- 2. Kennedy PC, Miller RB: The female genital system. In:
Pathology of Domestic Animals, eds. Jubb KVF, Kennedy PC, Palmer
N, 4th ed., Vol. 3, pp. 349-470. Academic Press, Inc., San Diego,
CA, 1993
- 3. Lawler DF, Evans RH, Reimers TJ, Colby ED, Monti KL:
Histopathologic features, environmental factors, and serum estrogen,
progesterone, and prolactin values associated with ovarian phase
and inflammatory uterine disease in cats. Am J Vet Res 52:1747-1753,
1991
- 4. Perez JF, Conley AJ, Dieter JA, Sanz-Ortega J, Lasley
BL: Studies on the origin of ovarian interstitial tissue and
the incidence of endometrial hyperplasia in domestic and feral
cats. Gen and Comp Endocrin 116:10-20, 1999
- 5. Potter K, Hancock DH, Gallina AM: Clinical and pathologic
features of endometrial hyperplasia, pyometra, and endometritis
in cat: 79 cases (1980 1985). J Am Vet Med Assoc 198:1427-1431,
1991
-
-
- J Scot Estep, DVM
Captain, United States Army
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: estep@afip.osd.mil
-
- * The American Veterinary Medical Association and the American
College of Veterinary Pathologists are co-sponsors of the Registry
of Veterinary Pathology. The C.L. Davis Foundation also provides
substantial support for the Registry.
- Return to WSC Case Menu
