Results
AFIP Wednesday Slide Conference - No. 17
January 19, 2000
- Conference Moderator:
Dr. Jerry L. Quance, Diplomate, ACVP
Maryland Department of Agriculture
Animal Health Laboratory
Frederick, MD 21702
-
- NOTE: Click on images for larger views. Use
browser's "Back" button to return to this page.
Return to WSC Case Menu
-
- Case I - UFSM-1 (AFIP 2687023)
-
- Signalment: Six-month-old, Holstein Friesian, female,
bovine.
-
- History: This is one of four 6-month-old heifers housed
in the same stall. There were 83 dairy cattle (Holstein Friesian)
in this farm. This heifer had normal temperature, loss of appetite,
apathy, incoordination, opisthothonus, and lateral recumbency.
It was euthanatized 5 days after the onset of clinical signs.
All cattle of this farm were tested with the skin mammal tuberculin
test and 50% were positive. Four months after the death of this
heifer, another 4-year-old cow died after showing similar neurological
signs for 2 days. Gross lesions found in the cow were similar
to those described here.
-
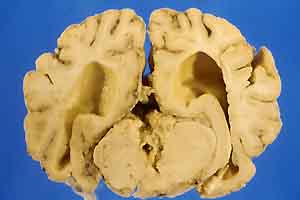
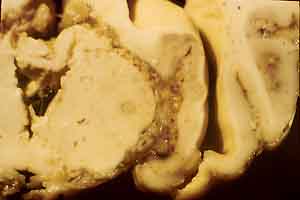
- Case 17-1. Gross Brain (see comments in text).
-
- Gross Pathology: The necropsy was performed at the
farm by the submitting veterinarian. Reportedly, no gross changes
were found in organs other than the brain. Gross examination
of the brain revealed increased cerebrospinal fluid (CSF), coning
of the cerebellum and flattening of cerebral gyri. The leptomeninges
were markedly thickened mainly over the cerebellum, base of the
cerebrum, and occipital cortex. The mesencephalic aqueduct was
blocked by cellular and fibrin exudate and dilation of lateral
ventricles (internal hydrocephalus). The fixed brain was sent
to our laboratory.
Contributor's Diagnoses and Comments: Thalamus, hippocampus
and rostral colliculi, granulomatous meningoencephalitis and
encephalitis with Langhans giant cells, caseous necrosis, mineralization,
vasculitis, and thrombosis.
-
- Etiological diagnosis: Bacterial meningitis and encephalitis.
Etiology: Mycobacterium sp.
-
- Morphological lesions are typical of bovine tuberculosis.
Lesions are more pronounced in the meninges but extension into
the brain through the vascular spaces occur. In some slides well
developed granulomas are seen within brain substance. Granulomatous
vasculitis associated with fibrinoid necrosis and occasional
thrombi are observed. Ziehl Neelsen stain revealed acid fast
organisms associated with these lesions. Although bacteriological
culture was not performed, the distribution of lesions suggests
that M. bovis is the most likely species of Mycobacterium
involved.
-
- Tuberculosis is endemic in Brazil. A survey carried out in
237 dairy herds showed that 0.66% of the tuberculin tested animals
were positive. In another survey carried out in abattoirs in
the state of Rio Grande do Sul, southern Brazil, in a period
of 8 years, 0.64% of slaughtered beef cattle had gross lesions
of tuberculosis. The prevalence of the disease is greater in
the north of the country. In areas with a high prevalence, bovine
tuberculosis is almost always caused by Mycobacterium bovis and
only when the control of the disease is improved, M.avium-intracellulare
is significant.
-
- The pathogenesis of tuberculosis has been extensively studied.
From a primary focus (known as primary complex) in calves the
disease disseminates in different ways: (i) by extension, forming
new tubercles; (ii) by lymphatics draining the primary complex
to the regional lymph nodes; (iii) by retrograde lymphatic route,
involving serosae; (iv) by lymphatic and hematogenous route,
through access to the thoracic duct and vena cava, causing miliary
tuberculosis, which is more common in the lung and less so in
the liver, kidney, and brain; (v) by direct hematogenous route,
which is common in the congenital form of tuberculosis, and (vi)
by dissemination through the bronchi.
-
- The distribution of the lesions of tuberculosis in the central
nervous system predominates in the meninges of the base of the
brain (basilar tuberculous meningitis). From there, the lesions
may extend to the submeningeal nervous tissue. Most likely, the
organism enters the brain by hematogenous route and is retained
in the choroid plexus and meningeal vessels reaching the CSF
and the ventricular system. Meningeal lesions are similar to
those seen in serosae and can readily disseminate through the
meningeal space; necrosis is usually more prominent in the meninges
than in other organs. The infection can be congenital and it
is usually found in young animals.
In cattle, the route of entry for M. bovis is usually the respiratory
(by aerosols) and digestive (by ingestion of contaminated food,
swallowed bronchial Mycobacterium containing exudate) systems;
lesions are localized in the mesenteric lymph nodes. Cutaneous,
congenital and genital are uncommon routes of entry for Mycobacterium
sp.
-
- In areas where the disease is common, up to 0.5% of the infection
in newborn calves occurs through the umbilicus. As uterine tuberculosis
is more common in cows than in any other species, congenital
tuberculosis is very common in calves. Other than congenital
infections, respiratory and digestive are the main routes in
young animals. Abattoir surveys show that the prevalence of tuberculosis
lesions in the nervous system is 0.35% to 0.53% while prevalence
in other organs is as follows: lungs, 60.9%; liver 30.7%, peritoneum
24%, kidney 6%, udder 4.6%, reproductive system 7.5%, bones 3.8%.
-
- Tuberculosis affecting the nervous system can have four presentations:
(i) meningeal, (ii) isolated tubercles in the brain, (iii) spinal
cord compression due to tuberculous osteomyelitis of the vertebral
bodies, and (iv) tuberculous neuritis due to extension of lesions
from other organs. The meningeal presentation is the most common.
The clinical signs can be insidious or abrupt in onset and include
fever, incoordination, apathy, seizures, blindness and opisthotonus.
Gross lesions are usually marked. There is opacity of leptomeninges,
and accumulation of fibrin-like exudate in the sulci, along the
blood vessels.
-
- AFIP Diagnosis: Cerebellum: Meningitis, granulomatous,
diffuse, severe, with necrotizing vasculitis, mineralization,
thrombosis, caseous necrosis, and multifocal granulomatous encephalitis,
Holstein Friesian, bovine.
-
- Conference Note: Mycobacterial infections are caused
by bacteria belonging to the family Mycobacteriaceae, order Actinomycetales.
Mycobacterium sp. are aerobic, weakly gram-positive, non-spore
forming, non-motile bacilli with wide variations in host affinity.
Mycobacteria stain with carbol dyes and resist subsequent decolorization
with inorganic acids. This characteristic which is due to the
spatial arrangement of mycolic acids within the cell wall makes
them acid fast.
-
- The ability of mycobacteria to survive and multiply within
macrophages determines whether disease will occur within the
host. Mycobacteria sp. utilize several virulence factors including
cord factor or trehalose dimycolate, surface glycolipid, sulfatides,
lipoarabinomannan, heteropolysaccharide, heat shock protein,
complement, and tubuloprotein. The types of immune responses
that are critical in responding to mycobacterial infection are
cell-mediated immunity and the delayed hypersensitivity response.
-
- The species causing "classic" tuberculosis are
termed the M. tuberculosis complex (MTC) and include M. bovis,
M. tuberculosis, M. africanum (rare cause of human
TB in Africa), and M. microti (a rodent pathogen that
has been reported to infect cats). Those species grouped together
causing the syndrome of M. avium complex (MAC), sometimes
referred to as "avian mycobacteriosis", include Mycobacterium
avium-intracellulare and M. avium spp. paratuberculosis.
The latter, which is the cause of Johne's disease in ruminants
(ruminant paratuberculosis), can also infect monogastric animals.
-
- Another separate group of myocobacterial infections is caused
by M. leprae and the disease is termed leprosy or Hansen's
disease. Feline and murine leprosy is caused by M. lepraemurium.
The final group, atypical mycobacteriosis, can be described as
localized opportunistic skin and subcutaneous infections caused
by saprophytic and rapidly growing mycobacteria, e.g. M. fortuitum,
M. chelonae, etc. Ziehl Neelsen acid fast stains provided
by the contributor demonstrated small numbers of acid fast bacilli.
-
- Contributor: Universidade Federal de Santa Maria,
Departamento de Patologia, 97119-900, Santa Maria, RS, Brazil.
-
- References:
- 1. Andrade GB, Riet-Correa F, Mielke PV, Méndez MC,
Shild AL: Estudo histológico e isolamento de micobactérias
de lesões similares à tuberculose em bovinos no
Rio Grande do Sul. Pesq. Vet Bras :81-86, 1991
- 2. Correa, CNM, Correa WM, Spago N, Matsumoto T: Tuberculose
nervosa em vaca leiteira. Arq Esc Vet UFMG 32(2):265-269, 1980
- 3. Dahme E. Nervensystem Besonderer Formen bakterieller Infektionen.
In: Grundriß der speziellen pathologischen Anatomie der
Haustiere, eds. Dahme E, Weiss E, 4 ed., p. 535. Ferdinand Enke,
Stuttgart, Germany, 1988
- 4. Dungworth DL: The Respiratory System. In: Pathology of
domestic animals, vol 2, eds. Jubb KVF, Kennedy PC, Palmer N,
4th ed., pp. 539- 699. Academic Press San Diego, 1993
- 5. Francis J: Bovine tuberculosis including a contrast with
human tuberculosis. pp. 63-125. Stamples Press, New York, NY,
1947
- 6. Frauchingen E, Hofmann W III: Die Erkrankungen der Hüllen
des Zentralnervensystems. In: Die Nervenkrankheiten des Rindes,
Frauchinger E, Hofmann W, pp.217-247. Hans Huber, Bern, Switzerland,
1941
- 7. Guedes RMC, Nogueira RHG, Facury Filho EJ, Lago L A: Meningite
tuberculosa bovina. Arq Bras Med Vet Zootec 49(1):131-135, 1997
- 8. Kantor IN: Regional and Country status reports. The Americas,
In: Mycobacterium bovis infection in animals and humans, eds.
Thoen CO, Steele JH, pp. 166- 202. Iowa State University, Ames,
Iowa, 1995
- 9. Palaske G: Ablauf und pathologische Anatomie der Tuberkulose
der verschiedenen Tierarten im besonderen. In: Pathologische
Anatomie und Pathogenese der spontanen Tuberkulose der Tiere.
ed. Pallaske G, p 54-99. Gustav Fischer, Stuttgart, 1961
- 10. Thoen C O, Himes E M: Mycobacterium. In: Pathogenesis
of Bacterial Infections in Animals, pp. 26-37, Iowa State University
Press, Ames, Iowa, 1986
- 11. Cotran RS, Kumar V, Collins T: Robbin's Pathologic Basis
of Disease, 6th ed., pp.332-351. W.B. Saunders, Philadelphia,
PA, 1999
-
-
- Case II - 99-4943 (AFIP 2694953 )
-
- Signalment: Tissues is from a 10-year-old, castrated
male, Thoroughbred horse.
-
- History: Owner reported horse had a swollen tongue
for a period of 2 weeks. Swelling continued for an additional
2 weeks, at which time a biopsy was taken from the cranial portion.
-
- Gross Pathology: A formalin-fixed, 1 x 1 x 2 cm piece
of tongue was received.
-
- Contributor's Diagnosis and Comments: Glossitis, granulomatous
and eosinophilic, multifocal, with intralesional sarcocysts.
The most significant changes are eosinophilic granulomas, accompanying
fibrosis, myofiber loss, and intramyofiber sarcocysts. Several
granulomas center on sarcocyst capsular fragments or degenerative
bradyzoites. Sarcocyst capsules have radial striations and are
approximately 2 mm thick.1 The sarcocysts are presumptively identified
as Sarcocystis fayeri, based on morphology and geographic location
(S. fayeri is the only species of sarcocyst reported in equine
muscle in the US).1,2 Sarcocystis sp. are obligate, coccidian
parasites that undergo asexual reproduction in the vascular endothelium
and myocytes of herbivorous intermediate hosts. Horses become
infected by consuming feed contaminated with sporocysts from
definitive hosts (dogs). Sarcocystis sp. infection rarely results
in myositis, although cases similar to this one have been sporadically
reported.2
-
- AFIP Diagnosis: Tongue: Granulomas, eosinophilic,
multiple, with myodegeneration, necrosis, regeneration, and intralesional
and extralesional protozoa, Thoroughbred, equine, etiology consistent
with Sarcocystis sp.
-
- Conference Note: Conference participants unanimously
agreed with the diagnosis of Sarcocystis myositis. Over 90 species
of Sarcocystis have been recognized in mammals, birds, and reptiles,
and at least 14 of these are regularly found in striated muscle
or the intestine (as a form of alimentary disease caused by the
sexual stages in the definitive, carnivorous host) of domestic
animals. Often clinical disease does not occur. The severity
of clinical signs varies with the species of parasite, the age
of the infected animal, and the number of sporocysts ingested.
-
- All Sarcocystis species have an obligatory two-host life
cycle. Definitive hosts are carnivores, which are usually clinically
unaffected. They prey on the herbivorous intermediate hosts.
Upon being ingested by carnivores and released from mature cysts,
zoites invade the intestinal epithelium and develop into gamonts.
Fertilization occurs, followed by the formation of oocysts, which
sporulate within the carnivore's intestine. Infective oocysts
are shed in the feces. Susceptible herbivores then ingest oocysts
or sporocysts, and sporozoites are released in the intestine
and migrate into arterioles, where first generation merogony
occurs in endothelial cells. Merozoites released from meronts
undergo second generation merogony in capillary endothelium throughout
the body. Upon subsequent liberation, merozoites enter circulating
mononuclear cells and undergo endodyogeny (third generation merogony).
Finally, zoites from second and third generation meronts enter
the heart, skeletal muscle, or neural tissue (varies with species)
and develop into immature noninfective sarcocysts containing
unicellular metrocytes. Metrocytes produce bradyzoites that are
infective for the definitive host, and whose presence characterizes
a mature sarcocyst.
-
- Contributor: Department of Veterinary Microbiology
and Pathology, Washington State University, Pullman, WA 99164-7040.
-
- References:
- 1. Cawthorn RJ, Clark M, Hudson R, Friesen D: Histological
and ultrastructural appearance of severe Sarcocystis fayeri infection
in a malnourished horse. J Vet Diagn Invest 2:342-245, 1990
- 2. Gardiner CH, Fayer R, Dubey JP: An Atlas of Protozoan
Parasites in Animal Tissues, 2nd ed., pp. 41-47. Armed Forces
Institute of Pathology, Washington, DC, 1998
- 3. Hulland TJ: Muscles and Tendons. In: Pathology of Domestic
Animals, vol. 1, eds. Jubb KVF, Kennedy PC, Palmer N, 4th ed.,
pp. 257-260. Academic Press, San Diego, CA, 1993
- 4. Traub-Dargatz JL, Schlipf JW Jr., Granstrom DE, Ingram
JT, Shelton GD, Getzy DM, Lappin MR, Baker DC: Multifocal myositis
associated with Sarcocystis sp. in a horse. JAVMA 205:1574-1576,
1994
-
-
- Case III - N 358/94 (AFIP 2506092)
-
- Signalment: 15-month-old female red deer (Cervus elaphus).
-
- History: This hind was from a herd of 50 farmed deer.
It was one of four similarly affected deer from this herd. All
four deer developed ill-thrift and intermittent diarrhea while
remaining bright and alert. This hind was euthanized.
-
- Gross Pathology: Numerous pinhead sized white foci
were scattered throughout the liver. The wall of the small intestine
was thickened particularly in the area of the terminal ileum,
and the luminal surface of both large and small intestine exhibited
transversely arranged rugae. The mesenteric lymph nodes were
marked enlarged and there was an associated lymphangitis.
-
- Laboratory Results: Numerous acid-fast bacilli were
seen in smears made from material taken from the mucosa of the
large and small intestine and from the lymph nodes. Mycobacterium
paratuberculosis was isolated on cultural examination of the
mucosa.
-
- Contributor's Diagnoses and Comments: Granulomatous
hepatitis, enteritis and lymphadenitis associated with M. paratuberculosis
infection.
-
- There are numerous small granulomatous foci randomly scattered
throughout the liver. These granulomas are composed of foamy
macrophages admixed with occasional lymphocytes. Large numbers
of acid-fast organisms were seen to be associated with these
granulomas on examination of Ziehl-Neelsen-stained sections (see
transparency). Histopathological examinations of other organs
confirmed the presence of a granulomatous lymphadenitis and enteritis
also associated with the presence of acid-fast organisms. Johne's
disease is uncommon in cattle and sheep in Ireland. However,
it has emerged as a significant condition in farmed red deer.
Onset of clinical disease may occur in animals as young as one-year-
old. In addition, lesions may occur in organs such as the lung
and the liver as well as in the intestine and its associated
lymph nodes.
-
- AFIP Diagnosis: Liver: Hepatitis, granulomatous, portal
and multifocal, moderate, with intrahistiocytic bacteria, red
deer (Cervus elaphus), cervid.
-
- Conference Note: Mycobacterium spp. are aerobic, facultative
intracellular, weakly gram-positive, non-spore forming, non-motile
bacilli with wide variation in host affinity. The bacterial cell
wall is composed of complex lipids including glycolipids, lipopolysaccharides,
lipoproteins, and waxes. Mycolic acid is the lipid that confers
the acid-fast property.
- Mycobacterium avium, ssp. paratuberculosis causes
chronic granulomatous ileitis/colitis, with associated villus
atrophy, and regional lymphadenitis (Johne's Disease) in ruminants.
-
- Paratuberculosis is a disease of major economic importance
to the dairy and beef cattle industries. In addition to cattle,
natural disease occurs in sheep, goats, and llamas, and has been
described in white-tailed deer, red deer, bighorn sheep, Rocky
Mountain goats, and other wild ruminants. Some strains are specific
for goats and non-pathogenic to cattle.
-
- Paratuberculosis occurs worldwide and appears to be spreading
insidiously. Channel Island breeds (Jersey and Guernsey) and
beef shorthorn cattle and some breeds of sheep may be more susceptible.
Since many infected cows appear healthy, and there is no reliable
antemortem detection method, the disease is easily spread. Although
only 1-2% of a herd may be clinically ill, 40-100% may be infected.
Cattle of an involved herd are divided into four categories:
1) infected with clinical signs; 2) asymptomatic shedders; 3)
asymptomatic nonshedders; 4) non-infected.
-
- The organism survives in the environment for 6-9 weeks. Calves
are usually infected by 3-6 months of age by ingesting contaminated
feces. In older animals macrophages can restrict intracellular
growth of bacteria, although lysis does not occur, conferring
age-dependent resistance to clinical disease. Organisms cross
the intestinal mucosa and enter macrophages in Peyer's patches
and local lymph nodes. Gross and histologic lesions are usually
confined to the ileum, large intestine and draining lymph nodes,
but the infection is systemic. In fulminating infections, bacteremia
may occur. Bacteria are shed mainly in feces, although organisms
may be excreted in milk, semen, urine and uterine secretions.
Bovine fetuses may be infected as early as the second month of
gestation. Similarly, embryos within the uterus of superovulated
cows can be infected, as the bacteria have been shown to adhere
to ova. This infection then may be spread to surrogate cows.
-
- M. avium ssp. paratuberculosis has been hypothesized
to be the cause of Crohn's disease in man. Crohn's disease is
a granulomatous condition affecting the lower ileum and often
the colon. Although lesions are somewhat similar, convincing
evidence of a causal association has not been produced.
-
- Contributor: Dept. of Veterinary Pathology, Faculty
of Vet. Medicine, University College Dublin, Shelbourne Road,
Ballsbridge, Dublin 4, Ireland.
-
- References:
- 1. Barker IK, Van Dreumel AA: The Alimentary System. In:
Pathology of Domestic Animals, eds. Jubb KVF, Kennedy PC, Palmer
N. 4th ed., vol 2, pp. 247-252. Academic Press, New York, NY,
1993
- 2. Power SB, Haagsma J, Smyth DP: Paratuberculosis in farmed
red deer (Cervus elaphus) in Ireland. Vet Rec 132(9):213-6, 1993
- 3. Van Kruiningen HJ: Lack of support for a common etiology
in Johne's disease of animals and Crohn's disease in humans.
Inflamm Bowel Dis 5(3):183-91, 1999
- 4. Williams ES, Snyder SP, Martin KL: Pathology of spontaneous
and experimental infection of North American wild ruminants with
Mycobacterium paratuberculosis. Vet Path 20:274-291, 1983
-
-
- Case IV - 99-231 (AFIP 2694980)
-
- Signalment: 4-year-old, male, black Labrador, dog.
-
- History: Dog originally from California. Presented
to the veterinary teaching hospital for epistaxis and respiratory
distress. While hospitalized the dog went into respiratory arrest
and died.
-
- Gross Pathology: Numerous, 10 cm long x 0.3-cm diameter,
white worms were in the right ventricle and caudal vena cava.
The intimal surface of the proximal pulmonary artery was irregular
with a granular appearance. The heart was not enlarged. All lung
lobes were mottled light to dark red.
-
- Laboratory Results: A moderate leukocytosis, characterized
by neutrophilia with a left shift, monocytosis and eosinophilia,
was present. Microfilaria morphologically consistent with Dirofilaria
immitis were seen on blood smears.
Contributor's Diagnoses and Comments:
- 1. Angiotrophic lymphoma, lung
2. Microfilaremia
The most significant change is the accumulation of neoplastic
round cells within the pulmonary vasculature. Neoplastic cells
widely extend into vessel walls, and occasional vessels are completely
occluded. Intravascular aggregates of fibrin are also evident,
both associated and unassociated with neoplastic cells. Immunohistochemical
stains using a polyclonal primary antibody directed against the
T-lymphocyte marker CD3 were performed on replicate sections
and CD3 antigen was identified in neoplastic cells. Small numbers
of microfilaria are among erythrocytes within blood vessels,
including septal capillaries.
-
- Angiotrophic lymphoma, also known as malignant angioendotheliomatosis,
is a rare neoplastic disorder described in humans, dogs, and
cats. Using immunoglobulin markers, neoplastic cells have been
identified as either of B- or T- cell origin. The proliferation
of neoplastic cells within the vasculature results in thrombosis
and tissue infarction, which often are responsible for presenting
clinical signs. The central nervous system and skin are common
sites in which neoplastic cells are initially identified, although
any tissue can be affected.
-
- In the present case, neoplastic cells were identified in
kidney, lung, pituitary gland, spleen, esophagus, liver, nasal
mucosa, prostate, multiple lymph nodes, intestine, thyroid gland,
adrenal gland, pancreas, and brain. Skin was not examined. In
affected animals, the clinical course is typically rapid, often
resulting in death (or euthanasia) within several weeks of the
initial diagnosis. Antemortem diagnosis is difficult, as no distinct
clinicopathologic or radiographic findings exist. Of special
note is that neoplastic cells do not circulate and consequently
are not evident on blood smears; hence biopsy of affected tissue
is frequently necessary to establish a diagnosis. In this case,
the clinical suspicion was that the dog died from pulmonary thromboembolism
secondary to dirofilariasis; the presence of the angiotrophic
lymphoma was unanticipated.
-
- AFIP Diagnosis:
- 1. Lung: Malignant lymphoma, intravascular, black Labrador,
canine.
2. Lung: Thrombosis, multifocal.
3. Lung: Arteriosclerosis, multifocal.
4. Lung: Microfilaria, intravascular, numerous.
-
- Conference Note: The term "angioendotheliomatosis"
has caused confusion as it refers to two very different vascular
lesions. One originally called "malignant angioendotheliomatosis"
has been found to be intravascular lymphoma. The other, "reactive
angioendotheliomatosis" has been reported in cats and humans
and is characterized by proliferation of endothelium and pericytes.
However, the feline and the human diseases are radically different:
the former is fatal and the latter is benign.
Conference participants agreed that there is significant vascular
sclerosis but could not determine whether this lesion was the
result of heartworm disease or intravascular lymphoma. Both have
been reported to cause this lesion. Immunohistochemical staining
for CD-3 and CD-79 performed at the Armed Forces Institute of
Pathology, failed to work on two attempts. Generally canine intravascular
lymphoma is of a T-cell origin, whereas most cases of intravascular
lymphoma in humans are B-cell lymphomas.
-
- Contributor: Department of Veterinary Microbiology
and Pathology, Washington State, University, Pullman, WA 99164-7040
-
- References:
1. Fuji R, Freels K, Summers B: Systemic reactive angioendotheliomatosis
in cats: Two cases and a review of the literature. ABSTRACT Vet
Pathol 35(5):420, 1998
- 2. Kilrain CG, Saik JE, and Jeglum KA: Malignant angioendotheliomatosis
with retinal detachments in a dog. JAVMA 204:918-921, 1994
- 3. LaPointe JM, Higgins RJ, Kortz GD, Bailey CS, Moore PF:
Intravascular malignant T-cell lymphoma (malignant angioendotheliomatosis)
in a cat. Vet Pathol 34:247-250, 1997
- 4. Perniciaro C, Winkelmann RK, Daoud MS, Su WP: Malignant
angioendotheliomatosis is an angiotropic intravascular lymphoma.
Immunohistochemical, ultrastructural, and molecular genetics
studies. Am Journal Dermatopathol 17:242-248, 1995
-
- J Scot Estep, DVM
Captain, United States Army
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: Estep@afip.osd.mil
-
- * The American Veterinary Medical Association and the American
College of Veterinary Pathologists are co-sponsors of the Registry
of Veterinary Pathology. The C.L. Davis Foundation also provides
substantial support for the Registry.
-
- Return to WSC Case Menu

