Results
AFIP Wednesday Slide Conference - No. 7
20 October 1999
- Conference Moderator:
COL Kelly Davis, Diplomate, ACVP
Pathology Division
U.S. Army Medical Research Institute of Infectious Disease
Ft. Detrick, Frederick, MD 21702-5011
-
- NOTE: Click on images for larger views. Use
browser's "Back" button to return to this page.
Return to WSC Case Menu
-
- Case I 3G (AFIP 2678957)
-
- Signalment: 5-year-old male rhesus monkey (Macaca
mulatta).
-
- History: Infected with simian immunodeficiency virus
(SIV) one year prior to euthanasia. Weakness, paraparesis before
euthanasia.
-
- Gross Pathology: No visible lesions.
-
- Contributor's Diagnoses and Comments:
- 1. Cauda equina, neuritis, suppurative and hemorrhage, multifocal,
acute, moderate.
- 2. Cauda equina, meningitis, suppurative and hemorrhage,
multifocal, acute, moderate.
- 3. Spleen and lymph node, lymphoid depletion, diffuse, chronic,
severe.
- 4. Liver, hepatitis, multifocal to coalescing, chronic, ongoing,
moderate with fibrosis and occasional intranuclear inclusions.
Occasional to numerous intranuclear inclusions were seen in macrophages
in the meninges in the cauda equina. Polymerase chain reaction
testing was performed on spinal cord tissue and was positive
for cytomegalovirus (CMV). The inclusions in the liver were also
typical of CMV. CMV is a common opportunistic infection in immunosuppressed
macaques. This SIV-infected macaque was immunosuppressed as suggested
by severe lymphoid depletion of the spleen and lymph nodes.
-
- AFIP Diagnoses:
- 1. Cauda equina: Neuritis and meningitis, necrohemorrhagic,
focally extensive, severe, with karyomegaly, cytomegaly and eosinophilic
intranuclear inclusion bodies and rare intracytoplasmic inclusion
bodies, rhesus monkey (Macaca mulatta), non-human primate, etiology
consistent with cytomegalovirus.
2. Nerve roots and ganglia: Ganglioneuritis, subacute, multifocal,
mild, with karyomegaly, cytomegaly and eosinophilic intranuclear
inclusion bodies and rare intracytoplasmic inclusion bodies,
etiology consistent with cytomegalovirus.
-
- Conference Note: Cytomegaloviruses are host-specific
members of the subfamily betaherpesvirinae within the herpesvirus
family. Man, non-human primates and a variety of other animals
are commonly infected. Generally, clinical disease is restricted
to immunocompromised individuals. Latent infection is common
in rhesus monkeys; immunosuppression often leads to clinical
disease.
-
- When clinical disease occurs, it tends to be disseminated.
In macaques infected with SIV, CMV most commonly affects the
lungs, lymph nodes, small intestine, liver and brain. Infection
generally produces characteristic karyomegalic cells with prominent
intranuclear inclusions often surrounded by a clear halo ("owl's
eye cells"). Rarely, smaller granular intracytoplasmic inclusions
may be found. Some sections contain cells with intracytoplasmic
inclusions of this type. Some conference participants thought
that these inclusions might be protozoal organisms.
Contributor: Division of Comparative Medicine, 459 Ross
Building, Johns Hopkins University, 720 Rutland Avenue, Baltimore,
MD 21205.
-
- References:
- 1. Baskin GB: Disseminated cytomegalovirus infection in immunodeficient
rhesus monkeys. Am J Pathol, 129(2):345-352, 1987.
2. Kuhn EM, Stole K, Matz-Rensing K, Mach M, Stahl-Henning C,
Hunsmann G, Kaup FJ: Immunohistochemical studies of productive
rhesus cytomegalovirus infection in rhesus monkey (Macaca mulatta)
infected with simian immunodeficiency virus. Vet Pathol 36(1):51-56,
1999
- 3. Murphy FA, Gibbs EBJ, Horzinek MC, Studdert MJ: Veterinary
Virology, 3rd ed., pp. 301-325. Academic Press, San Diego, CA,
1999
-
-
- Case II - 99-0581 (AFIP 2694784)
-
- Signalment: Adult, domestic shorthair, intact, female,
cat, Felis domesticus.
-
- History: This cat was exposed by the oronasal route
to 50,000 TCID50 of a non-plaque purified strain of Nipah virus
originally isolated from a human who died following a febrile
encephalitis. Six days post exposure the cat developed fever
and increased respiratory rate. Respiratory signs progressed
to severe dyspnea and the cat was euthanized on day 9.
-
- Gross Pathology: At necropsy the lungs did not collapse
on opening the thorax; the lobes were "wet" and had
a diffuse blotchy hemorrhagic appearance. There was purplish-red
consolidation of the right apical and intermediate lung lobes
and sanguinous frothy fluid in the bronchi. The tracheal mucosa
was hemorrhagic particularly at the thoracic inlet. Mediastinal
edema was noted together with increased (15ml) sanguinous free
pleural fluid. The carcass was dehydrated and slightly icteric.
-
- Laboratory Results: Nipah virus was isolated from
lung, tonsil, spleen, and urine. Positive reactions by immunoperoxidase
testing using rabbit anti-Hendra virus antibody were identified
in lung, meninges, lymphoid tissues and blood vessels (endothelium
and muscle). Specifically, immunostaining was noted in pulmonary
alveolar epithelium, epithelial syncytia, bronchiolar epithelium
and bronchoalveolar debris.
Contributor's Diagnosis and Comments: Lung: Focal to diffuse
acute alveolitis and vasculopathy with epithelial and endothelial
syncytia, edema, caused by infection with Nipah virus.
-
- Further histologic lesions included mild essentially non-suppurative
meningitis; individual cell necrosis of lymphoid cells; generalized
necrosis of lymphoid tissue (possibly a consequence of infarction);
syncytia formation in lymphoid tissue, particularly in paracortical
areas; and acute vasculitis with fibrinoid necrosis of vessel
walls in various tissues. Observation of immunopositivity to
anti-Hendra virus antibody in respiratory epithelium together
with airway debris suggests that, in contrast to Hendra virus,
Nipah virus may at least be transmitted by bronchial secretions.
-
- Between September 1998 and April 1999 over two hundred cases
of human febrile encephalitis (over 100 resulting in death) were
reported to the Malaysian Ministry of Health. These cases occurred
primarily in adult men with a history of close contact with swine.
At the same time a poorly defined disease syndrome causing both
illness (cough, dyspnea, tremors, irritability) and death was
noted in pigs from the same regions. Nine cases of human illness
also occurred in abattoir workers handling Malaysian pigs. Until
March 1999 the human disease was attributed to Japanese Encephalitis
when a novel paramyxovirus was isolated from one of the people
who had died.
-
- Initial characterization of the virus suggested that the
most closely related known virus was Hendra virus. The novel
virus, subsequently named Nipah virus, exhibited approximately
20% variation from Hendra virus at the nucleotide level and 10%
variation at the amino acid level for the first 2 genes studied.
Nipah virus was confirmed to be the etiologic agent of the disease
syndrome present in Malaysian pigs and an extensive program of
culling of pigs from infected properties was initiated.
-
- Further investigations on field samples from Malaysia have
indicated the host range for Nipah virus includes horses, cats,
bats, and probably dogs, as well as pigs and people. There is
an obvious parallel to Hendra virus in the developing understanding
of the role of bats in the epidemiology of Nipah virus in Malaysia.
-
- AFIP Diagnosis: Lung: Pneumonia, bronchointerstitial,
necrotizing, fibrinosuppurative and hemorrhagic, diffuse, severe,
with fibrin thrombi, epithelial and endothelial syncytia and
eosinophilic intracytoplasmic inclusions, domestic short hair,
feline.
-
- Conference Note: Nipah virus is a single stranded
RNA virus in the family Paramyxoviridae, subfamily Paramyxovirinae.
Paramyxoviruses are negative sense RNA viruses that require RNA
dependent RNA polymerase to be transcribed (transcription produces
mRNA from DNA or RNA, translation produces protein from mRNA).
Unlike orthomyxoviruses, paramyxoviruses have fusion protein
(F protein). F protein is required for paramyxoviral penetration
of host cells. Cellular proteases cleave F protein transforming
it into F1 and F2 that act on the plasma membrane of the cell
and cause fusion with the viral envelope. F protein is also important
in maintaining infection by causing cell to cell fusion and allowing
the virus to spread without exposure to neutralizing antibodies.
-
- Hendra virus, another recently described paramyxovirus, was
previously known as equine morbillivirus. In horses, Hendra virus
can induce pulmonary edema characterized by gelatinous distention
of the subpleural lymphatics. This gross appearance closely resembles
African horse sickness. Histologically, there is vascular degeneration
similar to equine viral arteritis, although endothelial syncytia
are present in Hendra virus disease and absent in equine viral
arteritis. Cats experimentally infected with Hendra virus can
develop lesions similar to those reported in Nipah virus pneumonia.
Contributor: CSIRO Animal Health, Australian Animal Health
Laboratory (AAHL), Private Bag 24 Geelong, Vic, Australia 3220
-
- References:
- 1. Anonymous: Exotic Animal Disease Bulletin, Nipah virus
in Malaysia: latest news from the Australian Animal Health Laboratory.
Aust Vet J 77:474-5, 1999
- 2. Hooper PT, Ketterer PJ, Hyatt AD, Russell GM: Lesions
of experimental equine morbillivirus pneumonia in horses. Vet
Pathol 34(4):312-322, 1997
- 3. Hooper PT, Westbury HA, Russel; GM: The lesions of experimental
equine morbillivirus disease in cats and guinea pigs. Vet Pathol
34(4):323-329, 1997
- 4. Paton NI, Leo YS, Zaki SR, Auchus AP, Lee KE, Ling AE,
Chew SK, Ang B, Rollin P, Umapathi T, Sng I, Lee CC, Lim E, Ksiazek
TG: Outbreak of Nipah-virus infection among abattoir workers
in Singapore. The Lancet 354:1253-1256
-
-
- Case III - 99030112 (AFIP 2685771)
-
- Signalment: 5-year-old, intact-male, English setter
dog.
-
- History: Animal presented with a 2-3 month history
of diarrhea. Dog is cachetic but has a ravenous appetite. Animal
has reportedly lost 20 pounds in past month.
-
- Gross Pathology: No abnormalities noted following
endoscopic examination.
-
- Contributor's Diagnosis and Comments: Granulomatous
enteritis with numerous intralesional and intracellular organisms
consistent with Histoplasma capsulatum.
- The intestinal villi are markedly widened and blunted by
numerous inflammatory cells that have infiltrated the lamina
propria. The macrophages contain numerous microorganisms characterized
by a central, 1-2 micron basophilic spherule surrounded by a
1 micron, circumferential, clear capsule (consistent with Histoplasma
capsulatum). Whereas the organisms predominate in the villi,
they are also present within macrophages located deeper at the
level of the crypts. Some sections exhibit hyperplasia of the
crypt epithelial cells.
-
- This case was exciting because of the tremendous numbers
of the Histoplasma present in nearly every section. They are
clearly visible without the need for special stains even at lower
magnifications. Lastly, this case represents one of the rare
rewards of making a significant diagnosis from an endoscopically
collected biopsy.
-
- AFIP Diagnosis: Small intestine: Enteritis, granulomatous,
diffuse, moderate, with numerous intrahistiocytic yeast, English
setter, canine, etiology consistent with Histoplasma capsulatum.
-
- Conference Note: Histoplasma capsulatum is
a dimorphic fungus. At 25°C it exists in the mycelium form
and at 37°C in the yeast form. Other dimorphic fungi include
Blastomyces dermatitdis, Sporothrix schenckii and
Coccidioides immitis. Dogs and cats are the two domestic
species that are most susceptible to infection, whereas birds
do not become infected due to their higher body temperature,
which prohibits fungal growth. The route of infection is by ingestion
or inhalation of microconidia from contaminated soil. Once inhaled
or ingested, the microconidia are phagocytized by macrophages
where there transform to yeast that reproduce by budding. The
yeast is then disseminated throughout the body within cells of
the monocyte-macrophage system.
-
- In dogs, histoplasmosis is often disseminated with frequent
gastrointestinal tract involvement, as in this case. In cats,
the disease is also often disseminated and produces a variety
of nonspecific clinical signs. Frequent clinicopathologic abnormalities
include nonregenerative anemia, neutrophilia, monocytosis, lymphopenia
and eosinophilia. Rarely, yeasts are present within polymorphonuclear
cells on peripheral blood smears.
-
- Histologic diagnosis is made by finding the thin walled,
2-4mm diameter yeast in tissue section, often within macrophages.
The yeast reproduce by single, narrow-based buds. The periodic
acid-Schiff reaction and fungal stains can be used to help identify
the organism. Other organisms that might be included in the differential
diagnosis include Leishmania sp., Toxoplasma gondii,
Cryptococcus neoformans, Blastomyces dermatitdis
and Sporothrix schenckii.
-
- Contributor: Oklahoma State University, College of
Veterinary Medicine, 250 VetMed, Stillwater, OK 74078.
-
- References:
- 1. Greene CE: Infectious Diseases of the Dog and Cat, 2nd
ed., pp. 378-372. WB Saunders Company, Philadelphia, PA, 1998
- 2. Jones TC, Hunt RD, King NW: Veterinary Pathology, 6th
ed. pp. 519-522, Williams and Wilkins, Philadelphia, PA, 1996
- 3. Valli VEO, Parry BW: The Hematopoietic system. In: Pathology
of Domestic Animals, vol. 3, eds. Jubb KVF, Kennedy PC, Palmer
N, 4th ed., pp. 247-249, Academic Press, San Diego, CA, 1993
-
-
- Case IV - 97-5113 (AFIP 2683740)
-
- Signalment: Bovine feedlot steer, age unspecified,
mixed breed beef.
-
- History: One month history of respiratory disease,
treated with multiple antibiotics including Micotil, Trivetrin
and Nuflor. Recent onset of hyphema and hypopyon (bilateral)
and uveitis. Chronic diarrhea and roughness in skin of interdigital
cleft. The animal was euthanized.
-
- Gross Pathology: Steer was in good nutritional condition.
Both eyes contained blood and cloudy white material in the anterior
chambers. Multifocal erosions were seen on the lateral surfaces
of the tongue. Interdigital skin was rough, but no distinct erosions
were found. The lungs were diffusely covered with fibrin, and
moderately firm adhesions were found between the lungs, diaphragm,
costal pleura and pericardial sac. Cranioventral portions of
both lungs were consolidated and contained a large number of
abscesses. Hilar lymph nodes were enlarged, red and edematous.
There were multifocal 1-5mm long white steaks throughout the
myocardium, and these were most numerous in the ventricles. A
single 5mm firm focus was seen in the free wall of the left ventricle.
All joints examined contained a small amount of fibrin.
-
- Laboratory Results:
- Clinical Pathology: CBC showed a neutrophilia with
a left shift, increased fibrinogen interpreted to be due to chronic
inflammation.
- Bacteriology: Lung: 4+ Arcanobacterium pyogenes. Immunology:
Heart: Bovine viral diarrhea virus POSITIVE, Lung: Mycoplasma
bovis POSITIVE.
-
- Contributor's Diagnoses and Comments:
Heart: Vascultis and perivasculitis, chronic, multifocal, severe.
Myocarditis, chronic, generalized, necrotizing, severe.
Myocardial parasitism, mild.
Lung (not submitted): Bronchopneumonia, chronic, locally extensive,
suppurative, severe with bronchiectasis.
Vasculitis and perivasculitis, generalized, severe, chronic.
Lymphoid depletion, moderate to severe, generalized.
-
- Vasculitis and perivasculitis were seen in nearly every organ
examined in this steer including: eyes, kidney, intestine, heart,
lung, and brain. Vessels were surrounded by or contained within
their walls a moderate infiltration of lymphocytes and plasma
cells. Frequently fibrinoid necrosis and hyaline degeneration
were seen in affected vessels. Affected vessels were positive
by immunohistochemistry for bovine viral diarrhea (BVD) virus.
Positive staining for BVD virus was also detected in atrophied
Peyers patches but was not present in the mucosa of the intestine,
or elsewhere in the body. Only A. pyogenes was cultured from
the lung of this animal, but lesions were positive for M. bovis
by immunohistochemistry. Lesions seen in the tongue of this animal
were typical of bovine papular stomatitis, rather than BVD.
-
- Vasculitis is an unusual lesion reported in animals with
mucosal disease, and has not been reported in the literature
to be associated with acute BVD infection. A single mention is
made in reference to this occurrence in association with clinical
laminitits in an adult bovine but the circumstances are not well
described. Animals with mucosal disease are reported to have
profuse diarrhea, wasting, and frequently severe enteritis, which
was not seen in this case. There have been some recent reports
documenting high mortality rates in cattle with acute BVD infection,
and this is speculated to be due to a strain with increased virulence.
Antemortem serology and virus isolation which may have helped
to differentiate these syndromes were not performed.
-
- Lesions in the heart and elsewhere were so severe, that a
differential diagnosis of malignant catarrhal fever was considered
in this case. Other animals with similar presentations and lesions
have been submitted for PCR for this herpesvirus and have consistently
been negative. Immunohistochemistry in this animal was positive
for BVD virus in affected vessels and in situ hybridization on
a limited number of cases with similar lesions have demonstrated
BVD virus exclusively in affected vessels.
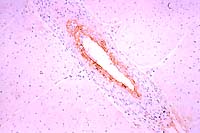 Immunoperoxidase
for BVD
Immunoperoxidase
for BVD
- Case 7-4. Heart. Note strong staining of arterial
endothelial cells for BVD antigens.
-
- The association of BVD with vasculitis and chronic pneumonia
involving M. bovis has been noted by pathologists in this laboratory
for several years. There are reports in the literature of BVD
being associated with other viruses and bacteria in the "shipping
fever complex" but no specific mention is made of M. bovis.
The specific interaction is unknown at this time but is speculated
to be immunosuppression and impaired leukocyte response. Pneumonia
involving M. bovis is often associated with a number of other
agents including Pasteurella sp. A. pyogenes, and occasionally
H. somnus. Many of the animals infected with M. bovis also show
a fibrinous arthritis and/or tenosynovitis (which was mild in
this case).
-
- AFIP Diagnosis:
- 1. Heart: Myocarditis, lymphoplasmacytic and histiocytic,
necrotizing, multifocal, moderate, with vasculitis and mineralization.
2. Heart: Sarcocysts, few.
-
- Conference Note: Bovine viral diarrhea virus is a
member of the order Flaviviridae, genus pestivirus. Flaviviruses
are 45-60nm, enveloped, icosohedral single stranded, positive-sense,
RNA viruses.
-
- Acute infection with BVD virus results in diarrhea in young
animals; with intrauterine infection, there is a wide range of
possible effects, depending on the age of the fetus and the nature
of the virus. Intrauterine infection with noncytopathic (based
on behavior in tissue culture) BVD virus before 80 days of gestation
often results in death and resorption or abortion; between 80-125
days, results vary from fetal abnormalities (retinal dysplasia,
cerebellar hypoplasia, hydrocephalus) to immunotolerance and
persistent infection. Previously it was thought that a second
infection with a cytopathic virus resulted in "mucosal disease",
but it has recently been discovered that this second infection
is actually a mutation from a noncytopathic to cytopathic biotype.
Clinically, mucosal disease is characterized by diarrhea, nasal
discharge, erosive and ulcerative stomatitis, dehydration and
eventual death. The pestivirus genus also includes hog cholera
virus and border disease virus in sheep. BVD virus infection
in pregnant sows can cause fetal death and resorption
- .
- A differential diagnosis for bovine viral myocarditis discussed
by conference participants included malignant catarrhal fever
(MCF) and rinderpest. Lesions of MCF are characterized by marked
perivascular and intramural infiltration of predominately large
lymphocytes with large nuclei and prominent nucleoli. There is
often an associated fibrinoid necrotizing vasculitis. The characteristic
inflammatory infiltrates and vascular changes occur in almost
all organs. Unlike rinderpest and BVD, the underlying lymphoproliferative
nature of MCF often causes a prominent lymphocytic hyperplasia
in multiple lymph nodes and prominent lymphoid follicles in the
splenic white pulp. The vascular lesions are more consistently
present and more severe in MCF than in BVD.
-
- Rinderpest, a morbillivirus of the family Paramyxoviridae,
causes necrosis of intestinal glands and Peyer's patches reminiscent
of the gastrointestinal lesions of BVD. Rinderpest may also cause
a necrotizing vasculitis; however, syncytial cells with eosinophilic
intracytoplasmic inclusions are often seen histologically in
cattle infected with rinderpest, and when present, distinguish
it from BVD and MCF.
-
- Contributor: Department of Veterinary Pathology, Western
College of Veterinary Medicine, University of Saskatchewan, 52
Campus Drive, Saskatoon, SK, S7N 5B4 Canada.
-
- References:
1. Baszler TV, Evermann J, Kaylor PS, Byington TC, Dilbeck PM:
Diagnosis of naturally occurring bovine viral diarrhea virus
infection in ruminants using monoclonal antibody-based immunohistochemistry.
Vet Pathol 32:609-628, 1995
- 2. Carmas S, van Dreumel T, Ridpath J, Hazlett M, Aves D,
Dubovi E, Tremblay R, Bolin S, Godkin A, Anderson N, Severe acute
bovine viral diarrhea in Ontario, 1993-1995. J Vet Diagn Invest
10:27-35, 1998
- 3. Jones TC, Hunt RD, King NW: Diseases caused by viruses.
In: Veterinary Pathology, 6th ed. Williams and Wilkins. pp. 299-302,
1997
- 4. Jubb KVF, Kennedy PC, Palmer N: The alimentary system.
In: Pathology of Domestic Animals, 4th ed., vol. 2, pp. 149-158,
Academic Press Inc., 1993
- 5. Murphy FA, Gibbs EPJ, Horzinek MC, Studdert MJ: Veterinary
Virology, 3rd ed. Pp. 29, 322-323, 563-566. Academic Press, San
Diego, CA, 1999
- 6. Richer L, Marois P, Lamontagne L: Association of bovine
viral diarrhea virus with multiple viral infections in bovine
respiratory disease outbreaks. Can Vet J 29:713-716, 1988.
- 7. Svensson C, and Bergsten C: Laminitis in young dairy calves
fed a high starch diet and with a history of bovine viral diarrhea
virus infection. Vet Rec 140:574-577, 1997
- J Scot Estep, DVM
Captain, VC, USA
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: estep@afip.osd.mil
-
- * The American Veterinary Medical Association and the American
College of Veterinary Pathologists are co-sponsors of the Registry
of Veterinary Pathology. The C.L. Davis Foundation also provides
substantial support for the Registry.
-
- Return to WSC Case Menu
 Immunoperoxidase
for BVD
Immunoperoxidase
for BVD