Results
AFIP Wednesday Slide Conference - No. 3
22 September 1999
- Conference Moderator:
LTC Mark Mense
Diplomate, ACVP
Walter Reed Army Institute of Research
Division of Pathology
Washington, D.C. 20307-5100
- NOTE: Click on images for larger views. Use
browser's "Back" button to return to this page.
Return to WSC Case Menu
-
- Case I - ND2 (AFIP2676133)
-
- Signalment: Three 10 to 14-day-old budgerigar ( Melopsittacus
undulatus) carcasses, gender unknown, were submitted to the North
Dakota State University Veterinary Diagnostic Laboratory.
-
- History: A budgerigar breeder began to experience
losses in the adult population with subsequent spread to the
newly hatched birds. New adults had recently been introduced
to the aviary. Diet included oat groats soaked in water, commercial
seed and water with a soluble vitamin added. Affected birds were
treated with broad-spectrum antibiotics and acyclovir.
-
- Gross Pathology: No significant gross abnormalities
were observed.
-
- Laboratory Results: Culture of small intestine and
lung yielded Enterococcus fecalis and Enterobacter amnigenus.
In situ hybridization tests performed on liver, kidney and heart
were positive for avian polyomavirus.
-
- Contributor's Diagnosis and Comments: Heart, myocardial
hemorrhage, coalescing, acute, severe with multifocal cardiac
myocyte degeneration and necrosis, and basophilic intranuclear
inclusion bodies due to avian polyomavirus.
-
- Avian polyomavirus (APV) is a member of the Papovaviridae
that causes a variety of microscopic lesions in a number of different
genera of psittacines and finches. Reported lesions include myocardial
and hepatic necrosis, splenic lymphoid atrophy, nephritis, ballooning
degeneration and acanthosis of the follicular epithelium, bone
marrow necrosis, and cerebral vasculitis. Affected cells frequently
contain characteristic, large, basophilic to amphophilic intranuclear
inclusion bodies. Death is typically acute and can involve high
numbers of birds; however, recovered individuals are thought
to become carriers. The virus resides in a latent state until
activated by periods of stress. The virus has a worldwide distribution,
and asymptomatic intermittently shedding adults maintain it in
avian populations. Molecular techniques such as PCR, DNA probes,
and in situ hybridization are available and provide a rapid,
sensitive, specific and economical means of providing diagnosis.
AFIP Diagnosis: Heart: Cardiomyocyte degeneration and
necrosis, multifocal, moderate, with hemorrhage, karyomegaly,
and amphophilic intranuclear inclusion bodies, budgerigar (Melopsittacus
undulatus), avian.
- Conference Note: Avian polyomavirus, also known as
budgerigar fledging disease virus, is a 40-50nm diameter, non-enveloped,
double stranded, DNA virus that is a member of the family Papovaviradae.
The virus can be transmitted vertically and horizontally. Viral
shedding occurs via secretions of the cloaca, skin, crop, respiratory
tract and urinary tract. Recovered birds can shed virus intermittently,
especially during periods of stress. Some birds have been known
to shed virus even in the presence of high serum antibody titers.
Embryos infected prior to the development of immunocompetence
may develop severe, fatal disease or a tolerant carrier state.
-
- Clinical signs generally depend on the age and condition
of the bird but can include: sudden death in chicks less than
10 days of age, reduced formation of down and contour feathers,
abdominal distention, subcutaneous hemorrhage, tremor of the
head and neck, ataxia and bleeding from feather follicles. Infection
has also been associated with decreased hatchability and embryonic
death.
-
- The most sensitive and specific method of diagnosis is identification
of DNA sequences by polymerase chain reaction (PCR) testing and/or
in situ hybridization. In the live bird, PCR can be performed
on material collected from the cloaca. With necropsy specimens,
liver and spleen are good tissues to test for viral DNA sequences.
- The differential diagnosis for cardiomyocyte degeneration
and necrosis in the budgerigar considered at the conference included
hypovitaminosis E, aflatoxicosis, lead intoxication and infection
with Chlamydia psittaci, avian polyomavirus, reovirus, adenovirus,
herpesvirus or psittacine beak and feather disease virus. The
presence of the large amphophilic intranuclear inclusions and
karyomegalic cells is characteristic of polyomaviral infection,
but similar inclusions may be seen in adenoviral infection. Specific
tests, such as PCR with DNA probes, are needed for definitive
diagnosis.
Contributor: North Dakota State University, Veterinary
Diagnostic Laboratory, Fargo, ND
-
- References:
1. Garcia AP, Latimer KS, Niagro FD, et al: Diagnosis of polyomavirus-induced
hepatic necrosis in psittacine birds using DNA probes. J Vet
Diag Invest. 6:308-314, 1994
- 2. Phalen DN, Wilson VG, Graham DL: Organ distribution of
avian polyomavirus DNA and virus-neutralizing antibody titers
in healthy adult budgerigars. Am J Vet Res. 54:2040-2047 1993
- 3. Ritchie BW, Harrison GJ, Harrison LR: Disease etiologies.
In: Avian Medicine Principles and Application. pp. 888-892, Wingers
Publishing Inc., Lake Worth Florida, 1994
- 4. Ritchie BW, Niagro FD, Latimer KS, et al: Avian polyomavirus:
An overview. JAAV. 5:147-153 1991
- 5. Ritchie BW, Niagro FD, Latimer KS, et al: Polyomavirus
infections in adult psittacine birds. JAAV. 5:202-206, 1991
-
-
- Case II - 99-1141 (AFIP 2679497)
-
- Signalment: A three-day-old, female, Quarterhorse
foal.
History: The foal was presented for abdominal distension
and umbilical edema. On physical exam, the foal had a capillary
refill time >3 seconds, was 8-10% dehydrated, and had abdominal
distension and umbilical edema. The foal was observed to urinate
normally. Abdominal radiographs showed a fluid density within
the peritoneal cavity and abdominal ultrasound showed a urine
filled bladder and free fluid within the peritoneal cavity. Two
liters of a turbid serosanguineous fluid was removed from the
peritoneal cavity. The foal died acutely.
Gross Pathology: A 20 cm in diameter, firm, irregular
mass was present in the peritoneal cavity associated with the
left liver lobe. The ventral aspect of the mass had an area of
capsular rupture with associated blood clots and necrotic neoplastic
tissue. On cut surface, the mass was mottled tan/red and friable.
Other findings included:
-Four liters of a red, watery, opaque fluid with multiple blood
clots present on peritoneal surfaces (hemoabdomen).
-Icterus.
-Umbilical edema.
-Acute pulmonary congestion and edema (agonal).
-
- Laboratory Results: The foal had a PCV of 15%, hypochloremia,
hyponatremia and azotemia (BUN=64, Cr=6.8).
- The peritoneal fluid had a PCV of 7%, TP of 3.0 mg/dl, BUN
of 62 and a Cr of 7.4.
-
- Contributor's Diagnosis and Comments: Liver, Hepatoblastoma
with hemorrhage and central necrosis.
-
- The hepatic mass consisted of two populations of cells arranged
in either tubules or trabeculae and pseudo-rosette formations.
The cell population within the well-developed tubular structures
was tall cuboidal with abundant eosinophilic cytoplasm, apical
brush borders, basilar vesicular nuclei with stippled chromatin
and occasional mitotic figures (< 1 per 20x field). This population
of cells was interpreted to have undergone ductal differentiation.
The second population of cells, which is more frequent, is arranged
in trabecular to pseudo-rosette structures. The cells are cuboidal
with lightly eosinophilic abundant cytoplasm with vesicular nuclei,
stippled chromatin and multiple nucleoli. Mitotic figures are
rare (1 per 20 field). The trabecular structures are separated
by a fine fibrous stroma. These cells are interpreted as "fetal
hepatic cells". There are multifocal areas of hemorrhage
and central necrosis within the mass. The neoplastic cell populations
were variably positive for cytokeratin (AE1/3) and neuron-specific
enolase (NSE) immunohistochemically. PAS staining with and without
diastase treatment revealed varying degrees of glycogen accumulation
within the neoplastic cells. There was no evidence of neuroendocrine
granules in either cell population by histochemistry (Churukian-Schenk
stain) or by electron microscopy.
Hepatoblastoma is a rare neoplasm in all species and has been
reported in young and adult sheep, mice, pigs, cattle and horses.
In humans, the neoplasm occurs usually within the first 2-3 years
of life. In general, hepatic neoplasia in the equine is quite
rare. Hepatoblastoma in the equine has been reported twice previously
in the literature (a 3-year-old Appaloosa gelding and a male
Thoroughbred fetus). The current theory of hepatoblastoma histogenesis
states that the neoplasm is derived from a hepatic pluripotential
stem cell. The stem cells can then undergo differentiation to
primarily embryonal hepatic cell types or occasionally to cartilage,
muscle, bone or neural tissue. Embryonal hepatic cell types are
described as polygonal cells with scanty basophilic cytoplasm,
large nuclei, a single prominent nucleolus, clumped chromatin
and numerous mitotic figures. These embryonal hepatic cells can
then undergo differentiation to fetal hepatic cell types or ductal
and/or squamous differentiation. Fetal hepatic cell types are
described as appearing similar to hepatocytes, but smaller with
eosinophilic and lightly granular cytoplasm, small oval nuclei,
1 to 2 small nucleoli and fine granular chromatin which are arranged
in trabecular structures. Ductal differentiation of embryonal
hepatic cells results in small duct formation, reminiscent of
bile ducts.
-
- AFIP Diagnosis: Liver: Hepatoblastoma, Quarterhorse,
equine.
-
- Conference Note: Conference participants and the Department
of Hepatic Pathology of the Armed Forces Institute of Pathology
concurred with the contributor's diagnosis of hepatoblastoma
based on the histomorphologic characteristics. In addition, immunohistochemistry
performed at the AFIP demonstrated that neoplastic cells are
multifocally positive for human hepatocyte antigen indicating
variable hepatic differentiation within the tumor. The differential
diagnosis discussed in conference included hepatoblastoma, cholangiocellular
carcinoma and metastatic carcinoma. In humans, prognosis is based
on the stage of disease. In the two reported equine cases, thoracic
metastasis was present. In mice, N-nitrosodiethylamine has been
shown to induce hepatoblastomas.
-
- Contributor: Department of Veterinary Biosciences,
The Ohio State University, 1925 Coffey Road, Columbus, OH 43210
-
- References:
1. Craig JR, Peters RL and Edmondson HA: Atlas of Tumor Pathology,
Tumors of the Liver and Intrahepatic Bile Ducts, Fascicle 26;
AFIP Washington D.C.: pp.190-7, 1988
- 2. Neu SM: Hepatoblastoma in an equine fetus. J Vet Diagn
Invest 5:634-637, 1993
- 3. Nonoyama T et al: Mouse hepatoblastomas: a histologic,
ultrastructural, and immunohistochemical study. Vet Pathol 25:
286-296, 1988
- 4. Nonoyama T et al: Hepatoblastoma with squamous differentiation
in a B6C3F1 mouse. Vet Pathol 23: 619-622, 1986
- 5. Prater PE, Patton CS, Held JP; Pleural effusion resulting
from malignant hepatoblastoma in a horse. JAVMA 194(3): 383-385,
1989
- 6. Shida T, Yamada T and Nomura Y: Hepatoblastoma in a dog.
J Vet Med Sci 59(12): 1167-1170, 1997
- 7. Shiga A, Shirota K, 2. Haas JE et al: Histopathology and
prognosis in childhood hepatoblastoma and hepatocarcinoma. Cancer
64: 1082-1095, 1989
-
-
- Case III - 96-2667 (AFIP 2683734)
-
- Signalment: Porcine, Yorkshire X Landrace
-
- History: 7 live pigs, 5-10 weeks of age, males and
females included.
Herd is porcine reproductive and respiratory syndrome (PRRS)
negative. Many other animals showing similar clinical signs in
herd. Early post-weaning period some of the piglets show poor
growth relative to herdmates. Develop pallor and heavy breathing.
Eventually pigs become icteric, emaciated and many die.
-
- Gross Pathology: Pigs in this submission showed a
wide range of lesions. All pigs were in poor body condition.
Most lymph nodes were enlarged, and lungs did not collapse. Multifocal
areas of lungs, especially cranioventrally, were mottled or red
and firm. Thymus of many pigs was small or could not be identified.
Kidneys were moderately to markedly enlarged. In one pig, the
kidneys were estimated to be approximately 15 times normal size.
Kidneys were soft, pale gray and appeared waxy. One pig showed
thinning of the wall of the small intestine, edema of the mesentery,
and the lumen contained a watery material.
-
- Laboratory Results: Positive for porcine circovirus
by immunohistochemistry.
-
- Contributor's Diagnoses and Comments:
- 1. Lymph node, follicular hyperplasia with histiocytosis
and eosinophil infiltration.
- 2. Lymph node, lymphocytolysis, moderate.
In many of the histologic sections, syncytial cells containing
amphophilic and occasionally basophilic intracytoplasmic inclusion
bodies can be seen in the cortex of the lymph node. The inclusion
bodies are most frequently noted in B-cell follicles (also see
kodachrome). Follicles are dominated by large lymphoblasts with
a decrease in the number of small lymphocytes found. Other lesions
in this group of pigs were: Lymphohistiocytic interstitial nephritis,
granulomatous lymphadenitis, interstitial pneumonia (often granulomatous)
with lymphoid hyperplasia, single cell necrosis in the exocrine
pancreas, and lymphohistiocytic infiltrates within the stomach
wall.
Immunohistochemistry positively identified porcine circovirus
type 2 in these tissues.
Porcine circovirus was first described as a contaminant in PK-15
cell lines. Antibodies to this virus are widespread in the pig
industry, and it has only been recently that this virus has been
implicated as a cause of significant disease. Postweaning multisystemic
wasting syndrome (PMWS) affects weaned pigs and is a progressive
disease with vague clinical signs including poor hair coat, weight
loss, jaundice, and dyspnea. Post mortem lesions are characteristic
for this disease and include generalized lymphadenopathy and
interstitial pneumonia. Histology of this disease is variable
with the stage. Early in the disease course, there is hyperplasia
of lymphoid tissue. As the disease progresses, infiltration of
lymphoid tissues with histiocytic cells, and gradual loss of
mature lymphocytes is seen. Syncytial cells are occasionally
seen within lymphoid tissues. Lymphohistiocytic infiltrates are
seen in multiple organs, including kidney, liver, lung, heart,
and intestine. Basophilic intracytoplasmic inclusion bodies characteristic
of circovirus are seen in many cases of PMWS. These are found
in histiocytic cells most frequently in B-cell follicles.
Porcine circovirus-associated disease is occasionally found in
conjunction with PRRS virus infection, and lesions produced by
these two viruses may be similar, particularly in the lungs.
Lymph node lesions seen in cases of PRRS tend to be more proliferative
and lack histiocytic infiltration. Lesions similar to naturally
occurring cases of PMWS have recently been reproduced by infection
of gnotobiotic pigs, and provide strong evidence that Porcine
circovirus is the cause of PMWS.
-
- AFIP Diagnosis: Lymph node: Lymphadenitis, granulomatous,
diffuse, moderate, with mild lymphoid hyperplasia and rare intrahistiocytic
polymorphous eosinophilic to amphophilic cytoplasmic inclusion
bodies, Yorkshire/Landrace cross, porcine.
-
- Conference Note: PMWS is an important emerging disease
in North America and Europe, consistently infecting pigs around
42 days of age. The disease is believed to be multifactorial.
Porcine circovirus (PCV) is a consistent factor in the development
of clinical disease; however, recent evidence suggests that porcine
parvovirus may also play a role. PCV is a non- enveloped, 15-24nm
diameter, single stranded, DNA virus of the family Circoviridae,
that replicates within the cytoplasm of the cell. Circoviruses
are the smallest viruses that infect vertebrates. Other member
of this family are chicken anemia virus, beak and feather disease
of psittacine birds and numerous viruses that infect plants.
There is little DNA homology among the three viruses that infect
vertebrates.
-
- Clinical signs: Wasting, dyspnea, enlarged lymph nodes,
diarrhea (profuse watery), pallor, and jaundice. Although jaundice
is a sporadic clinical sign, its presence is useful in differentiating
PMWS from PRRS.
-
- Gross lesions: Lesions vary somewhat based on the
stage of the disease, but commonly include: Lymph node enlargement
(particularly inguinal, mesenteric, bronchial, and mediastinal),
pallor or icterus of skin and mucous membranes, noncollapsing
to atelectatic lungs, diffuse atrophy and mottling of liver,
gastric ulceration, thin-walled edematous intestines, and occasionally
enlarged edematous kidneys.
-
- Histologic signs: The presence of polymorphous, botryoid,
basophilic intracytoplasmic inclusions within histiocytes (occasionally
multinucleate) that completely replace the B-cell region of lymph
node follicles is considered a unique feature of this disease.
Other lesions include: interstitial pneumonia; lymphohistiocytic
periportal hepatitis with hepatocyte degeneration; edema and
lymphohistiocytic, lymphoblastic peripelvic nephritis; lymphoid
depletion of spleen with replacement by histiocytes; lymphohistiocytic
gastroenteritis; and lymphohistiocytic pancreatitis.
Contributor: Department of Veterinary Pathology, Western
College of Veterinary Medicine, University of Saskatchewan, 52
Campus Drive, Saskatoon, SK, S7N 5B4 Canada
-
- References:
1. Allan GM, Kennedy S, McNeilly F, et al: Experimental reproduction
of severe wasting disease by co-infection of pigs with porcine
circovirus and porcine parvovirus. J Comp Path 121:1-11, 1999
- 2. Ellis J, Krakowka S, LairmoreM, Haines D, et al: Reproduction
of lesions of postweaning multisystemic wasting syndrome in gnotobiotic
piglets. J Vet Diagn Invest 11:3-14, 1999
- 3. Harding JCS, Clark EG: Recognizing and diagnosing postweaning
multisystemic wasting syndrome (PMWS). Swine Health and Production
5(5):201-203, 1997.
4. Harding JCS, Clark EG, Strokappe JH, Willson PI, Ellis JA:
Postweaning multisystemic wasting syndrome: epidemiology and
clinical presentation. Swine Health and Production 6(6):249-254,
1998
- 5. Rosell C, Segales J, et al: Pathological, immunohistochemical
and in-situ hybridization studies of natural cases of postweaning
multisystemic wasting syndrome (PMWS) in pigs. J Comp Path 120:
59-78 1999
-
-
- Case IV - 99-2 (AFIP 2680556)
-
- Signalment: Adult (1-2 years/age), wild-type zebrafish
(Brachydanio rerio)
-
- History: The fish was from a facility that contains
about 3,000 - 5,000 zebrafish housed in three independent, recirculating
freshwater systems. There was no previous history of clinically
observable infectious disease within the facility. A relatively
closed colony management system was in place with only bleached
embryos admitted into the facility.
Peracute gas-bubble disease (GBD) due to mechanical failure was
diagnosed three months earlier, which resulted in approximately
40% mortality of the fish within one life support system. Multiple
fish (survivors of GBD incident) began spontaneously developing
variably-sized ulcerative skin lesions along the flank caudal
to the opercula. Affected fish became progressively lethargic
and emaciated. There were no clinical signs among the fish in
the other two recirculating water systems. The following water
quality parameters were routinely measured and found to be within
normal limits: pH, temperature, ammonia, nitrite, nitrate, and
conductivity.
-
- Gross Pathology: Several fish demonstrated variably-sized,
spherical, ulcerative skin lesions along the flank caudal to
the opercula. Skin lesions ranged from superficial erosions to
deep ulcers. Affected fish also demonstrated pin-point hemorrhages
at the base of the fins and around the anal pore.
-
- Laboratory Results: Culture of liver, kidney, and
spleen from multiple fish on LJ (Lowenstein-Jensen) and 7H11
media were positive for Mycobacterium spp. Cultures exhibited
growth on the selective media between 10 - 21 days.
-
- Contributor's Diagnoses and Comments:
- 1. Oophoritis and peritonitis, severe, chronic, granulomatous
with caseous necrosis, egg necrosis, and intralesional acid-fast
bacilli, etiology: Mycobacterium.
- 2. Skeletal muscle necrosis and mineralization, mild-moderate,
multifocal.
Microscopic lesions in the zebrafish (Brachydanio rerio) consisted
of multifocal granulomatous oophoritis with necrosis and areas
of peritonitis. The ovary had variably-sized (approximately 0.1
to 1 mm-diameter) well-delimited granulomas as well as poorly
organized infiltrates of macrophages. Granulomas consisted of
closely spaced collections of macrophages, including numerous
epithelioid and foamy macrophages, and peripheral circumferential
bands of fibrosis. Many granulomas had necrotic centers with
coagulated anucleate or karyopyknotic cells, amorphous granular
debris, and, in large granulomas, remnants of collapsed egg walls
admixed with hypereosinophilic coagulum. Occasionally, extracellular
brown pigment accumulation and mineralization were also evident
in necrotic areas. Ziehl-Neelson staining demonstrated numerous,
intracellular and extracellular acid fast bacilli within most
granulomas. However, some granulomas lacked both acid fast bacilli
and areas of central necrosis, and others had central necrosis
with no discernible bacteria. Also present were unorganized infiltrates
of large, epithelioid macrophages in the ovary between or within
necrotic eggs. Multinucleate giant cells were infrequent. Similar
granulomatous inflammation was present within the peritoneal
connective tissue around portions of the intestine. Sporadic
skeletal muscle necrosis and mineralization were also seen.
-
- Atypical mycobacterial infections of fish are most commonly
associated with Mycobacterium marinum, M. fortuitum, or M. chelonae.
Due to its long incubation period and chronic, subclinical form,
this insidious disease can remain undetected within established
facilities for extended periods of time. Mycobacterial infections
of fish have been identified worldwide in over 150 species of
salt and fresh water fish. (Talaat et al, 1997). Once established,
Mycobacterium spp. can become a resident of the microbial flora
within the water system.
-
- The clinical signs of mycobacteriosis in zebrafish can be
highly variable since both acute and chronic forms of infection
have been characterized (Talaat et al, 1998). Chronically diseased
animals usually manifest by having a poor growth rate, chronic
wasting, and emaciation. There is usually an associated decrease
in reproductive rates and a slightly increased mortality rate
within the affected colony. Acutely diseased animals often demonstrate
the generalized clinical condition known as "dropsy syndrome"
which consists of abdominal distention, and scale edema. This
edema results in a lifting or "porcupine-like" effect
to the scales. Petechiation or ulceration of scales and fin erosion
are often evident.
-
- Preliminary diagnosis of mycobacteriosis is based on the
identification of clinical signs consistent with the disease.
Histologic examination of affected kidney, liver, and splenic
tissue often yields acid-fast positive staining, rod shaped bacteria
in affected tissues. The atypical aquatic Mycobacterium species
may display staining characteristics similar to gram-positive
bacteria. However, definitive confirmation of Mycobacterium infection
is currently made only by culture of the organisms on LJ (Lowenstein-Jensen)
and 7H11 media and subsequent biochemical analysis. Atypical
Mycobacterium spp. are extremely slow growing organisms in culture
and may require 30-60 days for definitive culture results to
be obtained. Frequently, acid fast bacteria may not be readily
identified histologically in affected tissues but yield positive
culture results when tested.
-
- Unfortunately, effective treatment of infected facilities
can only be accomplished by eradication of infected stocks and
subsequent disinfection of all substrates within the facility.
Various attempts at treatment with a number of antibiotics have
had limited success in controlling the infection but not eliminating
it. Since various Mycobacterium species have been isolated from
the environmental biofilms which form within water systems (Schultze-Röbbecke
et al, 1992), disinfection of the tank and filter system is necessary.
Prior to sanitation of the water system, all associated filter
material and disposable equipment should be discarded. Disinfection
of the water system and surfaces should be conducted with a bleach
solution. The outer surfaces of all tanks and related hardware
should be treated with the same bleach solution. Restock the
system and culture fish after several months to monitor for re-infection
of the system. It is important to realize that once an infection
has occurred within a facility, it is very difficult to completely
sanitize effectively.
-
- Atypical cutaneous mycobacterial infections of humans have
been well documented. Known as "fish handler's granuloma"
or "swimmer's granuloma," these infections are usually
self-limiting and result only in a localized area of erythema
and swelling on the affected extremity. However, more serious
clinical disease such as persistent cutaneous granulomas, osteomyelitis,
and tenosynovitis have been reported (Chang et al., 1999; Gatt,
1998; Murry, 1998; Shih et al., 1997) as a result of trauma with
infected surfaces or concurrent immunosuppression... Life-threatening
and fatal disease due to M. marinum and M. fortuitum have also
been documented (Lessing et al., 1993). Zoonotic transmission
of M. marinum and M. fortuitum to fish handlers or persons in
close contact with infected fish or aquaria has been documented.
Frequently, resolution of these persistent infections involves
lengthy systemic antibiotic treatment regimes (Hoyen et al.,
1998; Levendoglu-Tugal et al., 1998). All laboratory personnel
in contact with the fish or associated hardware should be aware
of the potential health risk. Precautionary measures, such as
the wearing of latex gloves, should be implemented when treating
outbreaks.

- Case 3-4.Gross
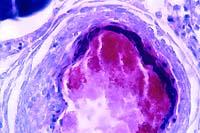 AFB
stain, 40x obj.
AFB
stain, 40x obj.
- Case 3-4.
-
- AFIP Diagnosis: Celom and ovary: Inflammation, granulomatous,
diffuse, moderate, with multiple granulomas, rupture of oocytes
and multiple colonies of bacilli, wild-type zebrafish (Brachydanio
rerio), pieces.
-
- Conference Note: Differential diagnosis discussed
for this case included infection by mycobacteria and nocardia.
Culture or other specific techniques are needed for definitive
diagnosis.
-
- Contributor: Massachusetts Institute of Technology
, 77 Massachusetts Avenue, Cambridge, MA. 02139
References:
1. Chang WJ, Tse DT, Rosa RH Jr., Miller D: Periocular atypical
mycobacterial infections. Ophthalmology. 106:86-90, 1999
- 2. Conroy G, Conroy D: Acid-fast bacterial infection and
its control in guppies (Lesbistes reticulatus) reared on an ornamental
fish farm in Venezuela. Vet Rec. 13:177-178, 1999
- 3. Gatt R, Cushieri P, Scibberras C: An unusual case of flexor
sheath tenosynovitis. J Hand Surg. 23:689-690, 1998
- 4. Hoyen HA, Lacey SH, Graham TJ: Atypical hand infections.
Hand Clin. 4:613-634, 1998
- 5. Lessing MP, Walker DD: Fatal pulmonary infection due to
Mycobacterium fortuitum. J Clin Pathol. 46:271, 1993
- 6. Levendoglu-Tugal O, Munoz J, Brudnicki A, Fevzi Ozkaynak
M, Sandoval C, Jayabose S: Infections due to nontuberculous mycobacteria
in children with leukemia. Clin Infect Dis. 27:1227-1230, 1998
- 7. Murry PM: Septic arthritis of the hand and wrist. Hand
Clin. 4:579-587, 1998
- 8. Schulze-Robbecke R, Janning B, Fischeder R: Occurrence
of mycobacteria in biofilm samples. Tuber Lung Dis. 73:141-144,1992
- 9. Stoskopf M: Fish Medicine. W.B. Saunders. Philadelphia,
PA. 1993
- 10. Shih JY, Hsueh PR, Chang YL, Chen MT, Yang PC, Luh KT:
Osteomyelitis and tenosynovitis due to Mycobacterium marinum
in a fish dealer. J Formos Med Assoc. 96:913-916, 1997
- 11. Talaat AM, Reimschuessel R, Wasserman SS, Trucksis M:
Goldfish, Carassius auratus, a novel animal model for the study
of Mycobacterium marinum pathogenesis. Infection and Immunity.
66:2938-2942, 1998
- 12. Talaat AM, Reimschuessel R, Trucksis M: Identification
of mycobacteria infecting fish to the species level using polymerase
chain reaction and restriction enzyme analysis. Vet Microbiol.
58:229-237, 1997
-
- J Scot Estep, DVM
Captain, VC, USA
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: estep@afip.osd.mil
-
- * The American Veterinary Medical Association and the American
College of Veterinary Pathologists are co-sponsors of the Registry
of Veterinary Pathology. The C.L. Davis Foundation also provides
substantial support for the Registry.
-
- Return to WSC Case Menu

 AFB
stain, 40x obj.
AFB
stain, 40x obj.