Results
AFIP Wednesday Slide Conference - No. 13
December 9, 1998
-
- Conference Moderator: Dr. F.M. Garner, Diplomate,
ACVP
4416 Oak Hill Road
Rockville, MD 20853
NOTE: Click on images for larger views. Use
browser's "Back" button to return to this page.
Return to WSC Case Menu
- Case I - 92749 (AFIP 2642676)
- Three photos
- Despite the cooperation of the submitter, we regret that
we are unable to post submitter supplied images associated with
this case on the web because we have been unable to determine
which have been previously copyright protected, or to obtain
permission from the copyright holder to use those images here.
-
- Signalment: Twelve-week-old, male, golden Syrian hamster
(Mesocricetus auratus).
-
- History: Numerous adult hamsters in a breeding colony
of approximately 4,000 Syrian hamsters developed skin tumors,
with most of the affected animals being males four to five months
of age. Female animals developed tumors at 10 to 12 weeks of
age (after the first litter). Affected animals were immediately
removed from the population, and two of the hamsters were submitted
for necropsy. No data concerning spontaneous recovery of affected
animals in this population is available.
-
- Gross Pathology: Multiple skin tumors measuring up
to five millimeters in diameter were found on the chin, neck,
thorax, and abdomen. Identical tumors measuring up to two millimeters
in diameter were found at the mucocutaneous junctions of the
mouth.
-
- Laboratory Results: No clinical laboratory data are
available. Attempts at virus isolation in tissue cultures using
the hamster kidney cell line BHK 21 were unsuccessful.
-
- Contributor's Diagnosis and Comments: Cutaneous trichogenous
tumors, papova (polyoma)-virus induced.
-
- The cutaneous tumors are of trichogenous origin. The incidence
of the tumors within the affected population suggested a contagious
process of viral etiology. This suspicion was confirmed by electron
microscopy which revealed numerous, irregularly arranged, viral
particles measuring approximately 40nm within the nuclei of degenerate
epithelial cells; viral particles were also occasionally found
in the cytoplasm. The size of virions suggests infection with
a polyomavirus.
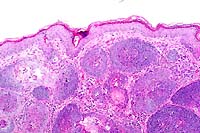 10x
obj
10x
obj
- Case 13-1. Skin. Numerous hair follicle-like structures
expand the dermis.
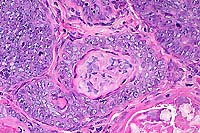 40x
obj
40x
obj
- Case 13-1. Dermis. There are glassy basophilic intranuclear
inclusions within more mature central cells of follicle.
- AFIP Diagnosis: Haired skin: Benign hair follicle
tumor, with few intranuclear inclusion bodies, golden Syrian
hamster (Mesocricetus auratus), rodent.
-
- Conference Note: An unencapsulated, well-circumscribed,
multilobulated neoplasm expands the dermis and subcutis, and
compresses subjacent skeletal muscle. The neoplasm is composed
of lobules of epithelial cells arranged in nests, broad cords
and hair follicle-like structures. A peripheral layer of basophilic
polygonal cells aligns along the basement membrane, and neoplastic
cells are more eosinophilic within inner layers of the abortive
hair follicles. Small numbers of intranuclear inclusions are
within the more mature, inner layers of neoplastic follicular
epithelium.
-
- The first spontaneous outbreak of polyomavirus-induced multicentric
cutaneous masses in hamsters was reported during the late 1960's
in the Buch golden Syrian hamster (HaB) colony in Berlin, Germany.
Tumors appear spontaneously in young animals between three months
and one year of age, and typically begin as miliary nodules on
the face, head and neck that progressively thicken and enlarge
to form coalescing masses in the skin and subcutis. The tumors
result from proliferation of the follicular epithelium, and often
contain central areas of keratin. Viral infection is thought
to occur by horizontal transmission. Viral particles identified
by electron microscopy are found in the nuclei of epithelial
cells immediately subjacent to the central areas of keratin,
but not within the more basal cells; the ultrastructural morphology
of virions is consistent with a polyomavirus (hamster polyomavirus
or HaPV). A closely related virus causing similar cutaneous neoplasms
in Syrian hamsters in Alabama has been reported.
-
- HaPV also induces lymphoma and leukemia in newborn animals
when injected percutaneously in a unique, essentially tumor free
colony of Syrian hamsters bred in Potsdam, Germany. While viral
particles are found within neoplastic epithelial cells in the
cutaneous form of the disease, neoplastic hematopoietic cells
are virus free but contain large amounts of extrachromosomal
viral DNA. Newborn mice may develop one of several types of hair
follicle tumors when inoculated with the Py (PTA) strain of murine
polyomavirus, but they do not develop hematopoietic neoplasia.
-
- The polyomaviruses and papillomaviruses are within the Papovaviridae
family. Papovaviridae are small, nonenveloped, icosahedral, double-stranded,
circular DNA viruses that measure between 45 - 55nm and replicate
in the nucleus. Members of the Papovaviridae are widely recognized
for their ability to cause proliferative or tumorigenic lesions
in various animal species. With oncogenic DNA viruses, viral
replication does not occur in transformed rapidly dividing cells,
and viral particles are not usually found in the germinal epithelium.
The cells of the upper or more differentiated layers permit viral
replication, and viral particles can be found in these areas;
on occasion, intranuclear inclusions can be seen by light microscopy.
This viral property explains the finding of intranuclear inclusions
within epithelial cells subjacent to the keratin elements in
the trichogenous tumors of the hamster.
-
- Contributor: Institute of Veterinary Pathology, University
of Munich, Veterinarstr. 13, 80539 Munchen, Germany.
-
- References:
- 1. Breur W, et al.: Papovavirus-induced trichogenous tumors
in Syrian hamsters (Mesocricetus auratus). J Vet Med 40:337-342,
1993.
- 2. Graffi A, et al.: Uber einen neuen virushaltingen Hauttumor
beim Goldhamster. Arch Geschwulstforsch 30:277-283, 1967.
- 3. Graffi A, et al.: Virus-associated skin tumors of the
Syrian hamster: Preliminary note. J Nat Cancer Inst 40:867-873,
1968.
- 4. Graffi I, et al.: Elektronenmikroskopische untersuchungen
uber das papova (papillom)-virus des Goldhamsters [Electronmicroscopic
investigations on papova virus of Syrian hamsters]. Arch Geschwulstforsch
40:191-236, 1972.
- 5. Scherneck S, et al.: The hamster polyomavirus - a brief
review of recent knowledge. Arch Geschulstforsch 60:271-278,
1990.
- 6. Prokoph H, Jandrig D, Arnold W, Scherneck S: Generation
of lymphoma-type variant hamster polyomavirus genomes in hamsters
susceptible to lymphoma induction. Arch Virol 142:53-63, 1997.
- 7. Jones TC, Hunt RD, King NW: Diseases caused by viruses.
In: Veterinary Pathology, 6th ed., pp. 251-257, Williams and
Wilkins, Philadelphia, 1997.
-
- Case II - RAT-1 (AFIP 2638256)
-
- Signalment: Two-year-old, male, Long Evans rat.
-
- History: The animal was humanely euthanized for necropsy
at the end of a two year carcinogenicity study. The animal was
in the control group.
-
- Gross Pathology: One testis was greatly enlarged (8cm
x 4cm x 4cm), oblong and soft. Sectioning revealed central necrosis
with cavitation. The other testis was normal. There were no other
gross findings.
Laboratory Results: Clinical pathology was within normal
limits.
-
- Contributor's Diagnosis and Comments: Seminoma, malignant.
-
- Histologically, neoplastic tissue replaced most of the testis,
with a few remaining atrophic and compressed tubules at the periphery
of some sections. The neoplastic cells were organized into tightly
packed sheets and large lobules divided by very fine fibrovascular
trabeculae with variably sized, scattered foci of necrosis that
often coalesced. Cells were large, and round to polygonal with
a round, normochromatic to hyperchromatic nucleus positioned
centrally or often eccentrically within a moderate amount of
eosinophilic, slightly granular cytoplasm. There was moderate
anisocytosis and anisokaryosis, and mitotic figures (which were
frequently bizarre) varied from 1 to 5 per high power field.
In many sections, clusters of neoplastic cells were seen within
peripheral blood or lymphatic vessels. Periodic acid-Schiff (PAS)
stains for glycogen revealed that most cells were negative with
slight patchy staining around the periphery of the nucleus in
some cells. Electron microscopy showed that the cells were very
undifferentiated and were not consistent with Sertoli cells or
Leydig cells, but contained some features of very early germ
cell differentiation. Immunohistochemistry revealed that the
cells were strongly positive for vimentin and S-100, but negative
for cytokeratins, neuron-specific enolase, placental alkaline
phosphatase and alpha fetoprotein.
-
- Testicular germ cell tumors in humans are divided into three
groups: seminomas, spermatocytic seminomas, and a mixed group
that includes embryonal carcinoma, teratoma, choriocarcinoma
and yolk sac carcinoma (Damjanov, 1990). The rat neoplasm was
not morphologically consistent with embryonal carcinoma, teratoma,
choriocarcinoma, or yolk sac carcinoma, and was negative for
cytokeratins for which most of these would stain positively.
However, there was also a lack of advanced spermatocytic differentiation
that would allow a definitive diagnosis of spermatocytic seminoma,
suggesting by exclusion the diagnosis of an undifferentiated
seminoma.
-
- Seminomas are extremely rare neoplasms in rats, in contrast
to humans and dogs. Indeed, one institution determined the incidence
within an historical data base to be 1 case in 31,868 animals
(0.003%) (Curtis, Bullock and Dunning, 1931). This is reflected
in a notable lack of literature about rat seminomas (Boorman
et.al., 1987). A recent search indicated that there were only
two articles in the literature describing the ultrastructural
and/or immunohistochemical features of rat germ cell tumors,
and these were of spermatocytic seminomas; one in a Fischer-344
rat (Nyska et. Al., 1993) and one in a Wistar rat (Kim, Fitzgerald
and De La Iglesia, 1985).
-
- Because of the lack of data, the biological behavior of these
neoplasms in rats is unclear. The large size, necrosis, anaplasia
and vascular invasion associated with this case suggest malignancy,
although no distant metastases were found.
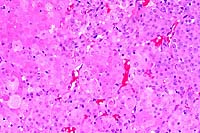 20x
obj
20x
obj 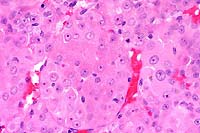 40x
obj
40x
obj
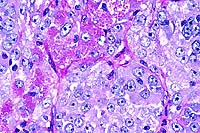
- Case 13-2. Testis. Neoplastic polygonal cells with
brightly eosinophilic globular cytoplasm form pockets & nests.
 40x
obj, PAS
40x
obj, PAS
- Case 13-2. Testis. Packeted polygonal tumor cells
rarely contain globular PAS positive cytoplasmic material.
-
- AFIP Diagnosis: Testis: Interstitial (Leydig) cell
tumor, malignant, Long Evans rat, rodent.
-
- Conference Note: Conference participants had great
difficulty with this case. While the majority agreed with the
contributor's diagnosis of malignant seminoma, several favored
malignant interstitial (Leydig) cell tumor. An unencapsulated,
lobulated, densely cellular neoplasm with multifocal to coalescing
areas of necrosis replaces most of the testicular parenchyma.
The neoplasm is composed of polygonal to elongate cells arranged
in nests, packets, and variably broad cords supported by a fine
to moderate fibrovascular stroma. A key histological feature
present in some sections is packets and lobules of large polygonal
cells that have abundant, brightly eosinophilic, granular to
globular cytoplasm and round, centrally placed nuclei with prominent
nucleoli; these cells are interpreted as neoplastic Leydig cells.
Cells with features intermediate between the Leydig-like cells
and the poorly differentiated cells are also present.
-
- This case was reviewed by the Department of Genitourinary
Pathology. They interpreted the neoplasm as of Leydig cell origin,
based primarily on the characteristic cells described above.
In humans, seminomas and interstitial (Leydig) cell tumors are
distinguished primarily based on histomorphology. Additionally,
human seminomas often contain glycogen and are frequently immunopositive
for placental alkaline phosphatase. In immunohistochemical studies
conducted at the AFIP, this tumor was negative for placental
alkaline phosphatase, and most cells were PAS-negative.
-
- An electron micrograph of this neoplasm published in a report
of this case shows ultrastructural features of steroid producing
cells, such as interstitial (Leydig) cells, including abundant
smooth endoplasmic reticulum, presence of a Golgi complex, lamellar
and tubular mitochondrial cristae, and intracytoplasmic lipid.
No feature diagnostic of seminoma is evident.
-
- This case demonstrates the difficulty in diagnosis of poorly
differentiated neoplasms. The malignant neoplasm in the rat testis,
like malignant Leydig cell tumors in humans, contains areas of
clonal differentiation. Only in scattered areas of the neoplasm
are there clearly identifiable features of Leydig cell differentiation.
Examination of multiple sections of poorly differentiated neoplasms
may be necessary for diagnosis. The presence of clonal differentiation,
high mitotic rate with bizarre mitoses, and intravascular neoplastic
cells warrants the interpretation of malignancy.
-
- Contributor: Pfizer Central Research, Department of
Drug Safety Evaluation, Bldg. 274, Eastern Point Road, Groton,
CT 06333.
-
- References:
- 1. Boorman GA, et al.: Seminoma, testis, rat. In: Monographs
on Pathology of Laboratory Animals, Genital System, Jones TC,
Mohr U, Hunt RD, eds., pp.192-198, Springer-Verlag, New York,
1987.
- 2. Curtis MR, Bullock FD, Dunning WF: A statistical study
of the occurrence of spontaneous tumors in a large colony of
rats. Amer J Cancer 15:67-121, 1931.
- 3. Damjanov I: Male reproductive system. In: Anderson's Pathology,
10th ed., Damjanov I, Linder J, eds., pp. 2166-2230, Mosby, St.
Louis, MO, 1990.
- 4. Kim SN, Fitzgerald JE, De La Iglesia FA: Spermatocytic
seminoma in the rat. Toxicol Pathol 13:215-221, 1985.
- 5. Nyska A, Harmelin A, Sandbank J, Scolnik M, Warner T:
Intratubular spermatocytic seminoma in a Fischer-344 rat. Toxicol
Pathol 21:397-401, 1993.
- 6. Kerlin RL, et al.: A poorly differentiated germ cell tumor
(seminoma) in a Long Evans rat. Toxicol Pathol 26:691-694, 1998.
- 7. McConnell RF, et al.: Proliferative lesions of the testes
in rats with selected examples from mice. In: Guides for Toxicological
Pathology, pp. 1-23, Society for Toxicological Pathology, American
Registry of Pathology, and the Armed Forces Institute of Pathology,
Washington DC, 1992.
- 8. Boorman GA, et al.: Interstitial cell tumor, testis, rat.
In: Monographs on Pathology of Laboratory Animals, Genital System,
Jones TC, Mohr U, Hunt RD, eds., pp.185-192, Springer-Verlag,
New York, 1987.
-
- Case III - C98-1029 (AFIP 2641899)
-
- Signalment: 1½ to 2-week-old male and female
Sprague Dawley rats.
-
- History: Three litters of Sprague Dawley rats ranging
in age from 1½ to 2 weeks developed diarrhea characterized
by yellow pasty to liquid feces. The perianal region of several
rats was stained with feces. Both sexes were equally affected.
-
- Gross Pathology: Gross examination of the infant rats
revealed distension of the small intestine and large intestine
with yellow fluid.
-
- Laboratory Results: Group D streptococcus was isolated
from the small intestine of affected rats.
- Contributor's Diagnosis and Comments: Small intestine: Streptococcus
enteropathy.
-
- Etiology: Streptococcus Group D.
-
- The bacteria lining the villi stained gram-positive and were
morphologically compatible with cocci. The diagnosis of streptococcal
enteropathy was based on:
1. Gram-positive cocci attached to the intestinal epithelium.
2. Streptococcus isolated as a predominate organism from the
small intestine.
3. Lack of inflammation or necrosis associated with the bacteria.
- The disease has been observed at our facility only twice
during the past sixteen years.
-
 40x
obj
40x
obj
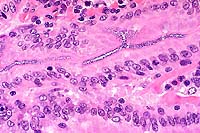
- Case 13-3. Small intestine. Abundant basophilic cocci
line the mucosal epithelium of some crypts.
-
- AFIP Diagnosis: Small intestine: Numerous luminal
epithelium adherent cocci, Sprague Dawley rat, rodent.
-
- Conference Note: Since 1985, there have been several
reports of an enteropathy in suckling neonatal rats caused by
enteric streptococci. Infected neonatal rats are often abnormally
small, have distended abdomens, poor haircoats, and fecal soiling
around the perineum. The disease is not associated with mortality.
Histologically, numerous epithelium adherent enterococci are
found along the intestinal villi with no other microscopic changes
or inflammation.
-
- Enterococci are normally considered commensal bacteria in
the intestine and are not thought of as etiologic agents of diarrhea.
There are several reports, however, of enterococcal diarrhea
in neonates of various domestic animal species including foals,
gnotobiotic piglets, chickens, and a puppy. In the horse, rat,
and pig, the most consistent histologic finding is the presence
of numerous Gram positive enteric cocci lining the epithelial
cells of small intestinal villi. Other microscopic changes noted
in the small intestine of the horse and pig include mild villus
atrophy and fusion, in contrast to the lesion in rats. The ability
of some species of enterococci to adhere to intestinal epithelium
is probably a key factor in the pathogenesis. The findings in
these reports seem to suggest that enterococci should be considered
potential etiologic agents of diarrhea in neonatal animals.
-
- Diarrhea and enteric disease of viral or bacterial etiology
occur uncommonly in the rat. Infectious diarrhea of infant rats
(IDIR), caused by a rotavirus, is characterized by diarrhea,
runting, and malabsorption in suckling rats less than twelve
days of age; the mortality rate is low. Potential bacterial causes
of gastrointestinal disease in rats include Clostridium piliforme,
the etiologic agent of Tyzzer's disease, and Salmonella sp.
-
- Contributor: Saint Jude Children's Research Hospital,
Comparative Medicine Division/ARC, 332 N. Lauderdale, Memphis,
TN 38105.
-
- References:
- 1. Hoover D, et al.: Streptococcal enteropathy in infant
rats. Lab Anim Sci 35:635-641, 1985.
- 2. Etheridge ME, Yolken RH, Vonderfecht SL: Enterococcus
hirae implicated as a cause of diarrhea in suckling rats. J Clin
Microbiol 26:1741-1744, 1988.
- 3. Etheridge ME, Vonderfecht SL: Diarrhea caused by a slow-growing
Enterococcus-like agent in neonatal rats. Lab Anim Sci 42:548-550,
1992.
- 4. Tzipori S, Hayes J, Sims L, Withers M: Streptococcus durans:
An unexpected enteropathogen of foals. J Infect Dis 150:589-593,
1984.
- 5. Harkness JE, Wagner JE: Specific diseases and conditions.
In: The Biology and Medicine of Rabbits and Rodents, 3rd ed.,
pp. 180-181, Lea & Febiger, Philadelphia, PA, 1989.
-
- Case IV - N97-136 (AFIP 2642346)
-
- Signalment: Young adult, mixed breed, male, canine.
-
- History: This animal appeared normal until the second
week of July when hair loss and foul-smelling skin were noted.
Skin scrapings did not reveal any agent, but mange was suspected.
There was no improvement after ivermectin treatment, and the
animal was euthanized.
Gross Pathology: The hair coat was thin over large portions
of the carcass, especially the flank, ventrum, axilla, groin,
and around the ears. Both ears had moderate crusting along free
borders.
-
- Contributor's Diagnosis and Comments: Moderate to
severe, chronic, locally-extensive dermatitis with intralesional
mites. Etiology: Sarcoptes scabiei var. canis.
-
- Multiple sections of skin from various body sites, including
the ears (section submitted), have changes ranging from acanthosis
with increased layers of surface keratin (orthokeratotic hyperkeratosis)
to focal zones of parakeratosis and cellular crusts. Moderate
numbers of bacterial colonies admixed within keratin can be seen
over the skin surface, as well as within a few of the keratin-plugged
hair follicles. The stratum corneum of the auricular skin has
various stages and sizes of mites (200-400um) consistent with
Sarcoptes sp. Inflammation within the subjacent dermis is mild
to moderate and consists of eosinophils and mast cells, with
fewer plasma cells and occasional macrophages. There is moderate
dermal fibrosis. The previously described inflammatory cells
occasionally surround other adnexal structures (apocrine glands
and sebaceous glands).
-
- Canine sarcoptic acariasis is an intensely pruritic, potentially
zoonotic, ectoparasitic infestation, the diagnosis of which relies
upon identification of the mite in skin scrapings and/or histologic
section. Varieties of Sarcoptes scabiei from different hosts
are highly host-specific, but morphologically indistinguishable.
-
 10x
obj
10x
obj
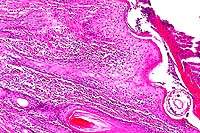
- Case 13-4. Skin. A serocellular crust overlies the
cross section of the mite. There is marked thickening of the
stratum spongiosum and corneum (acanthosis), intracellular edema
of corneal keratinocytes (hydropic degeneration), and a brisk
inflammatory infiltrate in the papillary dermis.
 40x
obj
40x
obj
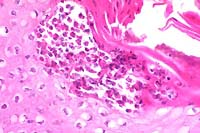
- Case 13-4. Note the subcorneal microabscess filled
with viable and degenerate eosinophils and neutrophils. The surrounding
keratinocytes are undergoing hydropic degeneration.
-
- AFIP Diagnosis: Haired skin, pinna: Dermatitis, eosinophilic,
mastocytic, lymphocytic, and plasmacytic, chronic, diffuse, moderate,
with hyperkeratotic crust, intracorneal microabscesses, epidermal
and follicular hyperplasia, parakeratotic and orthokeratotic
hyperkeratosis, and intracorneal mites, mixed breed dog, canine,
etiology consistent with Sarcoptes scabiei var. canis.
-
- Conference Note: Canine scabies is a highly contagious,
pruritic, skin disease caused by the epidermal mite Sarcoptes
scabiei var. canis. The mite is relatively host specific, though
the parasite may uncommonly cause transient pruritic skin disease
in other species, including humans and cats. Feline scabies is
caused by Notoedres cati, while Sarcoptes scabiei var. hominis
is the scabies mite of humans. Sarcoptes is a common and economically
important ectoparasite in swine, causing depressed growth rates
and decreased rates of food conversion. The disease also occurs
in cattle and goats, but is probably less important than psoroptic
mange.
-
- Sarcoptes completes its life cycle in tunnels burrowed into
the stratum corneum of the epidermis. The female mates with a
male in a molting pocket close to the skin surface and then burrows
through the stratum corneum to feed on cells of the stratum granulosum
and stratum spinosum over several weeks. The mite is equipped
with cutting mouthparts and cutting hooks on the legs. Epidermal
cell damage incites proliferative changes in adjacent keratinocytes,
and the tunnel openings at the skin surface become sealed with
a thick parakeratotic crust. The female lays her eggs in these
burrows, then vacates the tunnels to allow the eggs to hatch.
The eggs hatch and develop through larval and nymph stages to
reach maturity in 10-15 days, depending upon species. The lesions
of sarcoptic mange result from direct mechanical damage to keratinocytes,
irritant effects of parasite secretions and excreta, and the
hypersensitivity reaction that develops against the mite.
-
- The distribution of gross lesions in the dog result from
the preference of the mites to infect sparsely haired skin. The
ventral abdomen, margins of the pinnae, chest, and lateral elbows
are common sites for sarcoptic lesions. Erythematous maculopapular
eruptions with crusting alopecia accompanied by self-traumatic
excoriations develop in these areas. Occasionally, the disease
may become generalized in immunosuppressed dogs, and affected
animals have severe, thick, adherent crusts with marked alopecia.
-
- Microscopically, the epidermis is characterized by moderate
to severe acanthosis and variable spongiosis, with both orthokeratotic
and parakeratotic hyperkeratosis. Serocellular crusts may occur.
Rarely, cross-sections of mites (200-400mm) or their eggs (100-150mm)
are found within the superficial layers of the epidermis. Inflammatory
cells are often present in the dermis, with scattered eosinophils
and mast cells intermingled with perivascular infiltrates of
lymphocytes and macrophages, characteristic of a hypersensitivity
response. Neutrophils and plasma cells may be present in specimens
from sites of erosions, excoriations, and crusting due to trauma.
In dogs suffering from generalized scabies, numerous mites may
be found embedded in thick crusts.
-
- The differential diagnosis includes other causes of parasitic
allergic skin diseases, especially flea allergy dermatitis. Clinically,
the distribution of the lesions previously described may suggest
the diagnosis of sarcoptic mange. Also, the degree of epidermal
hyperplasia is generally greater in chronic sarcoptic mange than
in chronic flea allergy. Histologic lesions of sarcoptic acariasis
in which there is extensive parakerakeratosis and no mites may
resemble zinc responsive dermatosis.
-
- Contributor: Department of Pathobiology, School of
Veterinary Medicine, Tuskegee, AL 36088.
-
- References:
- 1. Morris DO, Dunstan RW: A histomorphological study of sarcoptic
acariasis in the dog: 19 cases. J Amer Anim Hosp Assoc 32:119-124,
1996.
- 2. Arlian LG, Morgan MS, Arends JJ: Immunologic cross-reactivity
among various strains of Sarcoptes scabiei. J Parasitol 82:66-72,
1996.
- 3. Arlian LG, Morgan MS, Rapp CM, Vyszenski-Moher DL: Some
effects of sarcoptic mange on dogs. J Parasitol 81:698-702, 1995.
- 4. Arlian LG, Runyan RA, Estes SA: Cross infestivity of Sarcoptes
scabiei. J Amer Acad Dermatol 10:979-986, 1984.
- 5. Yager JA, Wilcock BP: Perivascular dermatitis. In: Color
Atlas and Text of Surgical Pathology in the Dog and Cat, volume
1, pp. 60-61, Mosby-Year Book, London, England, 1994.
- 6. Gross TL, Ihrke PJ, Walder EJ: Perivascular diseases of
the dermis. In: Veterinary Dermatopathology, Reinhardt RW, ed.,
pp. 123-125, Mosby-Year Book, St. Louis, MO, 1992.
- 7. Yager JA, Scott DW: The skin and appendages. In: Pathology
of Domestic Animals, Jubb KVF, Kennedy PC, Palmer N, eds., 4th
ed., volume 1, pp. 681-682, Academic Press, San Diego, CA, 1993.
International Veterinary Pathology Slide Bank:
Laser disc frame# 04357, 04572, 04571, 04818, 09941, 18667, 18668.
- Ed Stevens, DVM
Captain, United States Army
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: STEVENSE@afip.osd.mil
-
- * The American Veterinary Medical Association and the American
College of Veterinary Pathologists are co-sponsors of the Registry
of Veterinary Pathology. The C.L. Davis Foundation also provides
substantial support for the Registry.
Return to WSC Case Menu
 10x
obj
10x
obj
 40x
obj
40x
obj
 20x
obj
20x
obj  40x
obj
40x
obj
 40x
obj, PAS
40x
obj, PAS
 40x
obj
40x
obj
 10x
obj
10x
obj
 40x
obj
40x
obj