Results
AFIP Wednesday Slide Conference - No. 5
30 September 1998
- Conference Moderator:
Dr. Terrance Wilson, Diplomate, ACVP
USDA Emergency Programs
Unit 41
4700 River Road
Riverdale, MD 20737-1231
NOTE: Click on images for larger views. Use
browser's "Back" button to return to this page.
Return to WSC Case Menu
Case I - 96/1255 (AFIP 2550628);
- one 2x2 histology color photo transparency
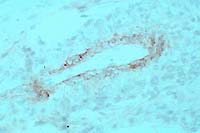
- Case 5-1. Immunohistochemical stain for BVD virus
structural & non-structural proteins
- Note positive staining of blood vessel tunica media.
-
- Signalment: A six-month-old, Brown Swiss calf was
presented to the clinic with signs of respiratory distress.
-
- History: Two calves from the same farm died a few
days previously. Upon admission, the calf was in poor body condition,
had a rectal temperature of 39.8°C, a heart rate of 60 beats
per minute, and a respiratory rate of 60 breaths per minute.
The animal coughed spontaneously. The clinicians detected loud
respiratory signs in the ventral lung fields. The muzzle was
dry, and the eyes were sunken. The calf was treated with NaCl-glucose,
Clamoxylä, Flumilarä, and Ventipulminä. The calf's
condition deteriorated over a five day period and was euthanized.
-
- Gross Pathology: The calf was thin. There were multiple
clumps of doughy, yellow, exudate in the tracheal lumen, and
the tracheal mucosa was reddened. The cranial lung lobes were
consolidated. On cut surface, a yellow-green, creamy mass was
seen in the bronchi and bronchioli. The ventral portions of all
left lung lobes were consolidated. All lung-associated lymph
nodes were enlarged and edematous. The heart and other organs
showed no macroscopic changes.
-
- Laboratory Results:
-
- Cytology Tracheobronchial wash:
- Macrophages: +
Neutrophils: +++
Bacteria: ++
Cell detritus: +++
-
- Bacteriology Tracheobronchial wash:
++/+++ Pasteurella haemolytica Biovar A
+ Leukocytes (microscopically)
-
- Radiography: A peribronchial interstitial pattern
could be seen in the dorsal areas of the lungs, and a bronchopneumonia
was detected in the ventral areas of the lungs.
-
- Parasitology: Eimeria, Trichostrongyles, and Strongylids
were detected.
-
- Hematology:
- 1. Hct: 20% (normal: 24-35%)
- 2. Hgb: 7.2 g/dl (normal: 8.3-11.7 g/dl)
- 3. Leukocytes: 2700/ml (normal: 4240-9090/ml)
- 4. Lymphocytes: 1782/ml (normal: 2192-5117/ml)
- 5. Fibrinogen: 16g/l (normal: 2-9 g/l)
-
- Histology: The following organs were histologically
examined: Lung; pulmonary lymph node; heart; liver; spleen; kidney;
intestine; and brain.
-
- Immunohistochemistry: Immunohistochemistry was performed
on snap-frozen sections of skin, thyroid gland, tongue and abomasum
collected at necropsy. Immunohistochemistry of the heart was
performed on paraffin-embedded tissue. Four monoclonal antibodies
against BVD-virus structural and non-structural proteins were
applied using the LSAB-method. All organs examined were positively
labeled. A positive control from a reference calf was run with
each batch of monoclonal antibodies. A negative control with
PBS (pH 8) was made with each slide.
- BVDV-LSAB
|
Organs |
Monoclonal Antibodies |
|
Ca3/34-C42 |
C16 |
C42 |
15c5 |
|
Skin |
+ |
++ |
+ |
++ |
|
Thyroid |
+ |
+ |
+ |
+ |
|
Tongue |
+ |
+ |
+ |
+ |
|
Abomasum |
+ |
+ |
+ |
+ |
|
Heart |
+ |
nd |
++ |
++ |
(nd= not done)
-
- Contributor's Diagnosis and Comments: Heart: Moderate
subacute perivasculitis and vasculitis. Mild multifocal subacute
myocarditis. Lung: Severe chronic bronchointerstitial pneumonia
with bronchiectasia and bronchiolitis obliterans (not submitted).
-
- The heart is submitted for Wednesday Slide Conference. The
perivascular region and the vessel walls are infiltrated with
mononuclear inflammatory cells. The endothelial cells are activated,
and in some regions there is a hyaline degeneration of the vessel
wall. This vasculitis was seen in the brain, intestine (vessels
of the submucosa), and the heart.
-
- The vasculitis is linked to a BVD-virus infection. It is
known that infection with this pestivirus can induce perivasculitis
and vasculitis with mononuclear inflammatory cells. These lesions
can be found in the intestine, the brain, the heart, the adrenal
cortices and other organs. Sometimes hyaline degeneration and
fibrinoid necrosis of the vessel walls in the submucosa of the
intestine and other organs can also be seen. Therefore, it is
difficult to differentiate vasculitis due to BVD-virus from that
seen in malignant catarrhal fever.
-
- BVD-virus can induce immunotolerance, persistent infection,
or mucosal disease. The course of the disease depends on the
time of infection (prenatal or postnatal) and the viral strains
involved. Due to BVD virus-induced immunosuppression, the calf
became susceptible to respiratory infection.

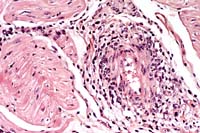
- Case 5-1. Immunohistochemical stain for BVD virus
structural & non-structural proteins
- Note positive staining of blood vessel tunica media.
 20x
obj.
20x
obj.
- Case 5-1. Heart: Note moderate influx of lymphocytes,
few histiocytes, and rare neutrophils within and around the vessel
wall.
AFIP Diagnosis: Heart, myocardium: Vasculitis and perivasculitis,
lymphohistiocytic and plasmacytic, multifocal, moderate, with
vascular fibrinoid necrosis, mild interstitial edema, and myocardial
necrosis, Brown Swiss, bovine.
-
- Conference Note: Sarcocysts are infrequently present
in some sections. Multifocal myocardial degeneration is also
present in some of the examined sections.
-
- Most conference participants agreed that fibrinoid necrosis
of vessels with perivascular distribution of inflammatory cells
in the myocardium is an unusual feature of BVD-virus (BVDV) infection.
Vasculitis has been frequently reported in arterioles of the
mesentery and intestinal submucosa. It has also been reported
in other organs, including the heart. A differential diagnosis
for bovine viral myocarditis discussed by conference participants
included malignant catarrhal fever (MCF) and rinderpest.
-
- Bovine viral diarrhea virus is a pestivirus which occurs
as cytopathogenic (cp) and noncytopathogenic (noncp) biotypes
based on their effects on tissue culture cells. The cytopathogenic
virus causes diarrhea in cattle exposed postnatally between six
months and two years of age. Clinically, the virus usually causes
a mild, acute, transient diarrhea with high morbidity. In some
cases, new strains of cpBVDV have caused outbreaks with high
mortality rates.
-
- Mucosal disease occurs in cattle that become infected in
utero with noncpBVDV. These animals develop a persistent infection
with the subsequent development of immunotolerance. Mucosal disease,
which is almost invariably fatal, generally develops in animals
between six months and two years of age and occurs when the noncpBVDV
is transformed to cpBVDV through RNA recombination. Superinfection
of cpBVDV may cause mucosal disease in persistently infected
animals if the cytopathogenic strain antigenically matches the
noncytopathogenic strain. Antigenically different cpBVDV strains
will not cause mucosal disease.
-
- Pestiviruses are enveloped, RNA viruses that measure 40 to
70nm in diameter. The pestivirus genus in the family Flaviviridae
also includes the viruses which cause hog cholera in swine and
border disease in sheep. Swine may be infected with BVDV, but
the virus does not cause clinical disease with the exception
of pregnant sows in which fetal death and resorption may occur.
-
- While the character and distribution of histologic lesions
may suggest a specific etiology, ancillary diagnostic tests and
procedures are often required to accurately diagnosis the various
bovine viral gastrointestinal and vesicular diseases. Virus isolation
requires labor intensive methods, prolonged periods of time,
and may fail to detect infection in a significant number of animals.
Recently developed immunohistochemical methods and polymerase
chain reaction tests provide practical, rapid means to accurately
confirm BVDV infection in animals.
-
- Rinderpest, a morbillivirus of the family Paramyxoviridae,
causes necrosis of intestinal glands and Peyer's patches reminiscent
of the gastrointestinal lesions of BVD. Rinderpest may also cause
a necrotizing vasculitis; however, syncytial cells with eosinophilic
intracytoplasmic inclusions are often seen histologically in
cattle infected with rinderpest, and when present, distinguish
it from BVD and MCF. Intranuclear inclusions may also occur within
the syncytial cells of rinderpest lesions.
-
- MCF, caused by a lymphotrophic gammaherpesvirus, causes a
marked perivascular and intramural infiltration of predominately
large lymphocytes with large nuclei and prominent nucleoli. There
is often an associated fibrinoid necrotizing vasculitis. The
characteristic inflammatory infiltrates and vascular changes
occur in almost all organs. Unlike rinderpest and BVD, the underlying
lymphoproliferative nature of MCF often causes a prominent lymphocytic
hyperplasia in multiple lymph nodes and prominent lymphoid follicles
in the splenic white pulp. The vascular lesions are more consistently
present and more severe in MCF than in BVD.
-
- Contributor: Institute of Veterinary Pathology, University
of Zurich, Winterthurerstr. 268, Zurich Switzerland 8057.
-
- References:
- 1. Kent TH, Moon HW: The comparative pathogenesis of some
enteric diseases. Vet Path 10:414-469, 1973.
- 2. Jubb KVF, Kennedy PC, Palmer N: The alimentary system.
In: Pathology of Domestic Animals, 4th ed., vol. 2, pp. 149-158,
Academic Press Inc., 1993.
- 3. Baszler TV, Evermann J, Kaylor PS, Byington TC, Dilbeck
PM: Diagnosis of naturally occurring bovine viral diarrhea virus
infection in ruminants using monoclonal antibody-based immunohistochemistry.
Vet Pathol 32: 609-628, 1995.
- 4. Thur B, Zlinsky K, Ehrensperger F: Immunohistochemical
detection of bovine viral diarrhea virus in skin biopsies: A
reliable and fast diagnostic tool. J Vet Med B43:163-166, 1996.
5. Jones TC, Hunt RD, King NW: Diseases caused by viruses. In:
Veterinary Pathology, 6th ed. Williams and Wilkins. pp. 299-302,
1997.
-
Case II - 96/558/4 (AFIP 2641092)
- Signalment: An adult horse.
-
- History: A horse was inoculated orally with 50,000
TCID50 equine morbillivirus/Hendra virus (EMV/HeV). Seven days
post inoculation, it developed tachycardia, anorexia, lethargy,
and increased respiratory rate. The horse deteriorated over the
next 24 hours and was euthanized.
-
- Gross Pathology: At necropsy there was marked pulmonary
edema with marked dilatation of lymphatics over the pleural surface
of the lung. All lymph nodes were congested. There were no other
gross post mortem lesions.
-
- Laboratory Results: Virus was isolated from the lung,
kidney, spleen, and urine. Lung, kidney, and many other tissues
were positive by indirect immunoperoxidase test using a rabbit
polyclonal serum to inactivated EMV/HeV.
-
- Contributor's Diagnosis and Comments:
- 1. Lungs: Edema, subacute (severe interlobular) and vasculopathy
with endothelial syncytia.
- 2. Kidneys: Vasculopathy with endothelial syncytia.
-
- The presence of syncytial endothelial cells in small and
medium-sized vessels in many organs is a diagnostic feature of
this disease, and in some lung sections in this case, is associated
with mural necrosis and lymphoid cell infiltration. Laboratory
methods demonstrated that viral antigen was confined to vascular
tissue and was readily identified in the tunica intima of arteries
and veins. The tunica media had positive immunostaining in a
smaller number of blood vessels. Positive immunostaining has
been observed in syncytial endothelial cells which are characteristic
of this infection.
In September 1994 in Hendra, a suburb of Brisbane, Australia,
infection with a previously undescribed member of the Paramyxoviridae
family resulted in the deaths of 13 horses and one human (the
adult male horse trainer) from an acute respiratory disease.
The virus was provisionally designated as equine morbillivirus,
but subsequent studies [3,4]; indicated the virus cannot be easily
classified in any of the existing genera in the family Paramyxoviridae.
Consequently, the virus has been renamed Hendra virus (HeV) [3,4,5].
-
- In October 1995, a farmer developed fatal encephalitis as
a result of HeV infection which was attributed to exposure to
two HeV infected horses that had died more than one year earlier
[7]. Extensive serological surveys throughout Queensland have
found no further evidence of HeV infection in horses or humans
[8,9]. However, fruit bats (flying foxes, Pteropus sp.) were
found to have a high prevalence of serological reactors to HeV
indicating they may be a wildlife reservoir of the virus [10].
Serological evidence of HeV infection has not been found in any
animal species other than fruit bats. In experimental studies,
guinea pigs and cats have been found to be susceptible to HeV
infection [11,12]. The lesions of HeV infection in horses have
been described by Hooper et al [13].
 10x
obj
10x
obj
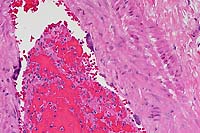
- Case 5- 2. Lung. The pleura and interlobular
septa are markedly expanded by clear space (edema) and scattered
lymphocytes an plasma cells. A pleural arteriole has fibrinoid
degeneration and necrosis of its walls with infiltrating and
adjacent lymphocytes and plasma cells.
 20x
obj.
20x
obj.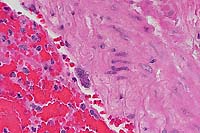 40x
obj
40x
obj
- Case 5- 2. Lung. Attached to the wall of this
pulmonary artery there are 3 endothelial syncytial cells. A 40x
objective view illustrates an eosinophilic intracytoplasmic inclusion
body separating the several of the nuclei of this cell.
 20x
obj
20x
obj
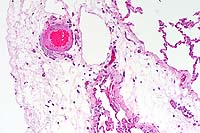
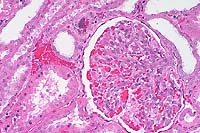
- Case 5- 2. Kidney. Note the syncytial endothelial
cell expanding the wall of a small arteriole adjacent to the
glomerulus.
AFIP Diagnosis:
- 1. Lung: Vasculopathy, characterized by endothelial syncytia,
mural necrosis, fibrinoid change, subacute perivasculitis, and
moderate interstitial edema, breed unspecified, equine.
- 2. Kidney, interstitial and glomerular blood vessels: Vasculopathy,
characterized by endothelial syncytia, mural necrosis, and fibrinoid
change.
-
- Conference Note: In some sections of lung and kidney,
rare eosinophilic intracytoplasmic inclusions are present within
endothelial syncytia. Mild degenerative changes of glomeruli
and tubular dilatation were also noted by some conference participants.
-
- The two recent outbreaks of a previously unrecognized equine
and human viral disease in Australia have sparked an intense
research effort to determine the pathogenesis, species susceptibility,
and reservoir of the virus. The disease in naturally infected
horses is characterized by an acute onset of respiratory distress,
anorexia, fever, depression, ataxia, and high mortality. As indicated
by the contributor, this is a zoonotic disease. The virus has
caused illness in three humans with two fatalities. Because of
the uncertain classification of this newly recognized virus,
it will be referred to in this text as equine morbillivirus/Hendra
virus (EMV/HeV).
-
- Experimentally, EMV/HeV causes lethal disease in horses,
cats, and guinea pigs, while dogs, mice, rats, chickens and rabbits
are refractory to infection. The lesion leading to death in experimentally
infected horses and cats is interstitial pneumonia with pulmonary
edema and accumulation of alveolar macrophages which develops
subsequent to virus-induced vascular changes. Intramural edema,
fibrinoid necrosis, endothelial syncytia, and perivascular mononuclear
cell inflammatory infiltrates characterize the vascular changes.
In horses, parenchymal lesions secondary to the vascular changes
more commonly occur in the kidney and brain, while in cats, histologic
changes in the gastrointestinal tract are more frequently observed.
(The authors of the initial experimental investigation of EMV/HeV
speculate that the variations in the distribution of lesions
may be due to limited sampling of the equine gut. The urgency
to establish the cause of the disease preempted an in-depth systematic
study of the virus in horses).
-
- The most significant gross lesion observed in experimentally
infected horses is pulmonary edema with subpleural lymphangiectasis.
The abundant frothy discharge present in the upper respiratory
tract in naturally infected horses did not occur in experimentally
infected horses; this may be due to environmental factors, various
treatments applied to field cases, and increased time of lesion
development in natural infections. In experimentally infected
cats, hydrothorax and pulmonary edema are the prominent gross
lesions with varying amounts of congestion and pulmonary hemorrhage.
-
- In guinea pigs the fundamental histopathologic finding is
fibrinoid degeneration/necrosis of small blood vessels surrounded
by a mononuclear inflammatory cell infiltrate, similar to horses
and cats. Additionally, the presence of endothelial syncytia
is common to all three of the susceptible species. Unlike horses
and cats, however, immunohistochemistry demonstrates that the
virus preferentially affects the larger vessels in guinea pigs
rather than the smaller vessels. This finding may explain the
lack of severe pulmonary edema in infected guinea pigs, the fatal
lesion of cats and horses. EMV/HeV does cause widespread vascular
disease in guinea pigs and is responsible for lesions in a variety
of organs. Cyanosis is observed grossly in infected guinea pigs
and is thought to be caused by myocardial insufficiency, failure
of the intercostal musculature, and/or failure of the lungs,
rather than pulmonary edema.
-
- Morbilliviruses are relatively large (150-250 nm), enveloped,
and contain single-stranded RNA. Examples of other morbilliviral
diseases include canine distemper, peste des petits ruminants,
phocine distemper, dolphin morbilliviral disease, and measles.
While interstitial pneumonia with syncytial cells is commonly
seen in morbilliviral infections, EMV/HeV is unique in that it
demonstrates a greater affinity for vascular tissues than the
other morbilliviruses. The other morbilliviruses, such as measles
and canine distemper, may infect endothelium but do not cause
the vascular lesions observed in EMV/HeV. This vascular tropism
occurs in both intravenously inoculated horses and subcutaneously
inoculated cats and guinea pigs, suggesting that this affinity
for blood vessels is real rather than a function of the route
of inoculation.
-
- Histologically, the vascular degeneration of EMV/HeV infection
resembles the changes observed in equine viral arteritis. Grossly,
African horse sickness causes pulmonary edema very similar to
that of EMV/HeV and should be considered in the differential
diagnosis.
-
- While investigators of the experimental equine cases noted
a histological absence of intracytoplasmic and intranuclear inclusions
within syncytia, a few conference participants identified rare
eosinophilic intracytoplasmic inclusions within endothelial syncytia,
especially within the renal vasculature. This histologic finding
is consistent with other morbilliviral infections.
-
- Contributor: Australian Animal Health Laboratory,
Ryrie Street, Geelong, Victoria, Australia 3219.
-
- References:
- 1. Murray PK, Selleck PW, Hooper PT et al.: A morbillivirus
that caused fatal disease in horses and humans. Science 268:94-96,
1995.
- 2. Selvey LA, Wells RM, McCormack JG et al.: Infections of
humans and horses by a newly described morbillivirus. Med J Aust
162:642-645, 1995.
- 3. Wang LF, Michalski W, Yy M, Pritchard LI, Crameri G, Shiell
B, Eaton BT: Novel P/V/C gene in a new Paramyxoviridae virus
which causes lethal infection in humans, horses and other animals.
J Virol 72:1482-1490, 1998.
- 4. Yu M, Hannsson E, Shiell B, Michalski W, Eaton BT, Wang
LF: Sequence analysis of the Hendra virus nucleoprotein gene:
Comparison with other members of the subfamily Paramyxovirinae.
J Gen Virol (in press).
- 5. Murray PK, Eaton B, Hooper P, et al.: Flying foxes, horses
and humans: A zoonosis caused by a new member of the Paramyxoviridae.
In: Emerging Infections 1, pp. 43-58, ASM Press, Washington D.C.,
1998.
- 6. O'Sullivan JD, Allworth AM, Paterson DL et al.: Fatal
encephalitis due to novel paramyxovirus transmitted from horses.
Lancet 349:93-95, 1997.
- 7. Hooper PT, Gould AR, Russell GM, Kattenbelt JA, Mitchell
G: The retrospective diagnosis of a second outbreak of equine
morbillivirus infection. Aust Vet J 74: 244-245, 1996.
- 8. Selvey L, Taylor R, Arklay A, Gerrard J: Screening of
bat carriers for antibodies to equine morbillivirus. Comm Dis
Intell 20:477-478, 1996.
- 9. Ward MP, Black PF, Childs AJ, et al.: Negative findings
from serological studies of equine morbillivirus in the Queensland
horse population. Aust Vet J 74:241-243, 1996.
- 10. Young PL, Halpin K, Selleck P et al.: Serological evidence
for the presence in pteropus bats of a paramyxovirus related
to equine morbillivirus. Emerg Infect Dis 2:239-240, 1996.
- 11. Westbury HA, Hooper PT, Selleck PW, Murray PK: Equine
morbillivirus pneumonia: Susceptibility of laboratory animals
to the virus. Aust Vet J 72:278-279, 1995.
- 12. Hooper PT, Ketterer PJ, Hyatt AD, Russell GM: Lesions
of experimental equine morbillivirus pneumonia in horses. Vet
Pathol 34:312-322, 1997.
- 13. Hooper PT, Westbury HA, Russell GM: The lesions of experimental
equine morbillivirus disease in cats and guinea pigs. Vet Pathol
34323-329, 1997.
-
Case III - 96-9621 (AFIP 2639838)
- Signalment: A seven-week-old, crossbred pig.
-
- History: This pig, from a multisource nursery facility
of about 700 animals, spontaneously developed an unusual dermatitis.
Two other pigs in this group developed similar lesions.
-
- Gross Pathology: Round to irregular, red to purple
macules and papules, often coalescing to form large irregular
patches and plaques, were present on the perineal area of the
hindquarters, limbs, ears, and ventral abdomen. There was subcutaneous
edema of the dependent sites.
-
- Laboratory Results: Bacterial cultures were negative.
Porcine respiratory and reproductive syndrome virus (PRRSV) was
detected in tissue homogenate samples by PCR and virus isolation.
Fluorescent antibody test was positive for porcine IgM and C3
within dermal blood vessels.
- Contributor's Diagnosis and Comments: Cutaneous vasculitis,
necrotizing and neutrophilic, with leucocytoclasia, thrombosis,
dermal hemorrhages and focal coagulative epidermal necrosis.
-
- Haired skin from the perineal area and from an ear are submitted.
A severe necrotizing vasculitis affecting small-caliber vessels
is characterized by infiltration of the vascular wall by neutrophils,
leucocytoclasia, fibrin exudation, and thromboses with reactive-hypertrophied
endothelial cells. In some sections these changes are best observed
in the deeper dermis and panniculus. Other cutaneous changes
include severe dermal hemorrhages, perivascular infiltration
of mononuclear cells and eosinophils, and coagulative necrosis
of the epidermis. Lesions found in other organs included bronchointerstitial
pneumonia, generalized reactive lymphadenopathy, and perivascular
cuffing of mononuclear cells in various tissues including skin.
-
- The cutaneous lesions in this case are part of a porcine
systemic vascular disease first recognized in the United Kingdom
[1,2], and subsequently in several countries including Canada
[3,4] and the United States [5]. Because of the frequent involvement
of the skin and kidneys among organs affected with the vascular
lesions, the disease was originally called porcine dermatitis/nephropathy
syndrome [1]. The disease affects mainly grower pigs and has
a low prevalence in swine herds. The prognosis of the condition
in affected pigs is dependent on the extent and the severity
of the vascular lesions found in internal organs, particularly
within the kidneys in which a severe exudative and necrotizing
glomerulonephritis may develop. The gross appearance and the
distribution of the cutaneous lesions in this animal are characteristic
of the disease in its acute stage.
-
- This vascular disease mainly involves small-caliber blood
vessels and appears to be immune-mediated [4,6]. The cause of
the condition is still undetermined, but it has been suggested
that PRRSV infection may play a role in the pathogenesis of this
systemic vascular disease of swine [4,7].
-
 20x
obj.
20x
obj.
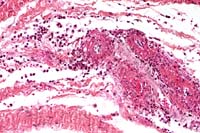
- Case 5-3. Dermis. There is a brisk infiltrate
of neutrophils and eosinophils around, infiltrating, and effacing
the necrotic walls of two parallel arterioles.
-
- AFIP Diagnosis: Haired skin: Vasculitis and perivasculitis,
necrotizing, neutrophilic and eosinophilic, acute, with multifocally
extensive dermal and subcutaneous hemorrhage, multifocal epidermal
necrosis, and mild epidermal hyperplasia, cross-bred pig, porcine.
-
- Conference Note: Porcine reproductive and respiratory
syndrome (PRRS) is a disease of pigs caused by an arterivirus
that commonly manifests as reproductive failure in sows, pneumonia
in young swine, and increased preweaning mortality. Additionally,
immunohistochemical studies suggest an association between PRRS
virus and a recently recognized systemic vasculitis primarily
affecting the skin and kidneys in young growing pigs, initially
termed dermatitis/nephropathy syndrome. In pigs with skin and
kidney lesions typical of the disease, viral antigen has been
detected in macrophages surrounding affected vessels. Through
the use of reverse transcription-polymerase chain reaction, PRRS
viral RNA has been detected in the lung and spleen of these animals.
-
- In addition to the gross lesions in the skin, the kidneys
may occasionally be swollen, pale, and contain numerous cortical
petechial hemorrhages, the result of an underlying necrotizing
vasculitis which occurs in the small and medium-sized vessels.
Pneumonia with generalized lymphadenopathy is usually observed
as well. The distribution of the cutaneous lesions described
by the contributor is typical for this entity. The acute cutaneous
lesions are hemorrhages due to necrotizing vasculitis, while
chronic lesions are brown crusts that cover ulcerated or excoriated
areas of skin.
-
- This systemic vascular disease of swine shares several similarities
with some of the human cutaneous vasculitides; leukocytoclastic
vasculitis in people occurs as hemorrhagic coalescing papules
and plaques often on dependent sites of the body such as the
legs and arms. Cutaneous necrotizing vasculitis often accompanies
systemic disease. While the skin may be the only organ affected,
other internal organs including the kidneys, central nervous
system, gastrointestinal tract, and joints, are often involved.
This is true in both pigs and humans. Systemic vasculitis may
occur from direct injury to vessels by infectious agents or by
immune-mediated mechanisms; most cutaneous vasculitides are thought
to be immune-mediated. Immune-mediated vascular damage may occur
through one of several mechanisms including formation or deposition
of immune complexes in vessels followed by cell-mediated or cytotoxic
antibody attack of vessels.
Several etiologies should be considered for erythema or skin
discoloration in pigs. Erysipelothrix rhusiopathiae, or swine
erysipelas, causes characteristic rhomboid or "diamond skin"
lesions acutely in pigs; the causative agent is a small, pleomorphic,
gram-positive bacterial rod that is sensitive to penicillin.
Bacterial septicemia caused by a variety of agents (Streptococcus
suis, Actinobacillus suis, A. pleuropneumoniae) may cause transitory
reddish discoloration of the skin. Additionally, salmonellosis,
Hemophilus parasuis, A. suis, and swine erysipelas may cause
cyanosis and congestion of the extremities with petechiation
and congestion in several organs. Porcine stress syndrome, associated
with handling or similar stresses in genetically predisposed
animals, may cause generalized blotchy blue or red discoloration
of the skin; usually there is also associated skeletal or myocardial
necrosis.
-
- Contributor: Department of Pathology and Microbiology,
Faculty of Veterinary Medicine, University of Montreal, C.P.
5000 St-Hyacinthe, P.Q. Canada J2S 7C6.
-
- References:
- 1. Smith WJ, Thomson JR, Done S: Dermatitis/nephropathy syndrome
of pigs. Vet Rec 132:47, 1993.
- 2. White M, Higgins RJ: Dermatitis/nephropathy syndrome of
pigs. Vet Rec 132:199, 1993.
- 3. Hélie P, Drolet R, Germain M-C, Bourgault A: Systemic
necrotizing vasculitis and glomerulonephritis in grower pigs
in southwestern Quebec. Can Vet J 36:50-154, 1995.
- 4. Thibault S, Drolet R, Germain M-C, D'Allaire S, et al.:
Cutaneous and systemic necrotizing vasculitis in swine. Vet Pathol
35:108-116, 1998.
- 5. Duran CO, Ramos-Varas JA, Render JA: Porcine dermatitis
and nephropathy syndrome : A new condition to include in the
differential diagnosis list for skin discoloration in swine.
Swine Health Prod 5:241-245, 1997.
- 6. Sierra MA, de las Mulas JM, Molenbeek RF, van Maanen C
et al.: Porcine immune complex glomerulonephritis dermatitis
(PIGD) syndrome. Europ J Vet Pathol 3:63-70, 1997.
- 7. Segales J, Piella J, Marco E, Mateu-de-Antonio EM, et
al.: Porcine dermatitis and nephropathy syndrome in Spain. Vet
Rec 142:483-486, 1998.
-
Case IV - A44522 (AFIP 2638306)
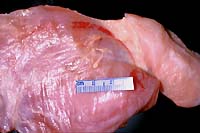
- Case 5-4. Gross. Overlying the muscle fascia there
are two yellowish-tan serpentine parasites with a bulbous end
and a slender body.
-
- Signalment: Tissues from adult feral hogs, Sus scrofa.
-
- History: The submitted tissues are from Florida feral
hogs that were trapped by local hunters in the Lake Okeechobee
and Placid area. After being transported to a Texas slaughter
establishment, they were slaughtered under United States Department
of Agriculture (USDA) inspection for human consumption. In April,
1998, while performing routine postmortem inspection of these
animals, the USDA veterinarian identified 1-3 cm, white to tan-yellow
lesions on the surfaces of the thoracic, thigh, and triceps musculature.
Some of the lesions and associated muscle were collected and
fixed in 10% neutral buffered formalin for histologic examination.
-
- Gross Pathology: Distributed on the surfaces of muscles
(abdominal, shoulder, thigh), and sometimes within the musculature
itself, were 1-3 cm, white to tan-yellow nodules. The nodules
were composed of a thin outer layer of connective tissue and
fat. Upon incision, the nodules contained a coiled, flat, slender,
white parasite, approximately 1 mm wide and up to 25 cm long.
The anterior end had a bulbous enlargement (2X3 mm) with a central
dimple or groove on the anterior aspect.
-
- Laboratory Results: None.
-
- Contributor's Diagnosis and Comments: Mild, multifocal
lymphoplasmacytic eosinophilic fasciitis with intralesional cestode
larvae, consistent with a pseudophyllidean plerocercoid.
-
- Histologically, there is a cystic space in the epimysial
connective tissue and fat. The space is surrounded by mild multifocal
infiltrates of eosinophils, lymphocytes, plasma cells, and macrophages.
The space contains single to multiple, closely bundled longitudinal
sections of a parasite. The parasite has irregular folds of pseudosegmentation
that is formed by a thick eosinophilic integument with inconspicuous
microvilli. There is an intervening layer of subintegumentary
cells that blend into a loose mesenchymal stroma that contains
calcareous corpuscles, longitudinal strips of smooth muscle,
and thin-walled excretory ducts.
-
- Sparganosis is an infection of tissues by the plerocercoid
stage of certain pseudophyllidean tapeworms. The plerocercoid
was originally called Sparganum, before the relationship to the
adult parasite was known. Adult stages of the parasite are typically
found in the intestine of domestic, feral, or wild canids and
felids, and eggs, rather than segments, are usually passed in
the feces. Water is required for maturation and development of
the ciliated coracidia from the egg, which is then followed by
ingestion of the coracidium by a copepod crustacean where it
develops into a procercoid. Due to this close relationship to
a water environment, common hosts for the final intermediate
plerocercoid stage include snakes and frogs. However, spargana
may develop in the tissues of essentially all vertebrates with
the exception of fish, by either ingestion of the procercoid
or plerocercoid stage.
-
- The importance of this infection lies in the serial transmission
of the plerocercoid in paratenic hosts, which may include food
animals and man. Spargana do not have distinct morphologic features,
and therefore they must be fed to a definitive host before taxonomic
separation into Spirometra and Diphyllobothrium species may be
attempted. Sparganosis, caused by Spirometra erinacei, is a well-defined
entity among local populations of feral hogs in Australia, where
expanded inspection procedures have been adopted when the parasite
is detected among animals slaughtered for human consumption.
The parasites are typically found in the connective tissues under
the peritoneum of the abdominal cavity, under the flare fat,
between abdominal muscles, under the skin of the inner aspect
of the hind legs, between the muscles of the hind legs, and under
the peritoneal lining of the abdominal organs and mesentery.
In contrast, report of infection of feral pigs in the United
States is rare.
-
- Grossly, the parasite may be mistaken for a small nerve or
blood vessel, and it may be easily overlooked unless the individual
is familiar with the disease and morphology of the parasite.
Consumption of undercooked or raw meat and the use of fresh tissues
as a poultice from an infected intermediate host have resulted
in human infections.
-
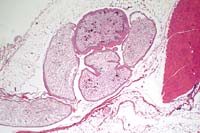 2x
obj.
2x
obj.
- Case 5-4. Muscle. Within the loose fibroadipose tissue
adjacent to skeletal muscle, there are multiple profiles of an
immature tapeworm (pleurocercoid), composed of loose mesenchyme
bearing abundant oval shaped calcified bodies (calcarious corpuscles).
-
- AFIP Diagnosis: Skeletal muscle and fibroadipose tissue:
Plerocercoid (sparganum), with multifocal chronic-active and
eosinophilic myositis and steatitis, feral hog (Sus scrofa),
porcine.
-
- Conference Note: As noted by the contributor, sparganosis
may be acquired by humans through the ingestion of undercooked
pork, in which the plerocercoid larvae remain alive, and through
the application of infected fresh animal tissue poultices, as
is practiced in some cultures. Frogs are most commonly used in
this practice. The sparganum invades the human tissue where the
poultice is applied, most often the eye. Additionally, humans
may contract the disease through drinking water contaminated
with infected copepods.
-
- A rare form of human sparganosis has been described in which
the infective plerocercoids proliferate and invade every tissue
except bone. Only nine human cases of this proliferating sparganosis
have been reported in which extensive invasion of the larvae
into lymphatics produces pronounced edema and an elephantiasis-like
syndrome. No confirmed animal cases of this atypical form of
infection have been reported. The underlying cause of this variant
of sparganosis is unknown but is believed to be aberrant forms
of spirometrids. In general, sparganosis is a relatively benign
human and animal disease; it may be more prevalent than reported
due to this benign nature.
-
- Contributor: United States Department of Agriculture,
Food Safety and Inspection Service, Office of Public Health and
Safety, P.O. Box 6085, Athens, GA 30604.
-
- References:
- 1. Daly JJ: Sparganosis. In: CRC Handbook Series on Zoonoses.
Section C, Vol. 1, pp. 293-312, CRC Press, Boca Raton, FL, 1982.
- 2. Dunn AM: Veterinary Helminthology, 2nd ed., pp. 129-291,
YearBook Medical Publishers, Inc., Chicago, IL, 1978.
- 3. Mueller JF: The biology of Spirometra. J Parasitol 60:3-13,
1974.
- 4. Appleton PL, Norton JH: Sparganosis: A parasitic problem
in feral pigs. Queensland Ag J 102:339-343, 1976.
- 5. Smith HM, Davidson WR, Nettles VF, Gerrish RR: Parasitisms
among wild swine in southeastern United States. J Am Vet Med
Assoc 181:1281-1284, 1982.
-
- International Veterinary Pathology Slide Bank:
Laser disc frame #3629
-
- Ed Stevens, DVM
Captain, United States Army
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: STEVENSE@afip.osd.mil
-
- * The American Veterinary Medical Association and the American
College of Veterinary Pathologists are co-sponsors of the Registry
of Veterinary Pathology. The C.L. Davis Foundation also provides
substantial support for the Registry.
Return to WSC Case Menu


 20x
obj.
20x
obj.
 10x
obj
10x
obj
 20x
obj.
20x
obj. 40x
obj
40x
obj
 20x
obj
20x
obj
 20x
obj.
20x
obj.

 2x
obj.
2x
obj.