Results
AFIP Wednesday Slide Conference - No. 3
16 September 1998
- Conference Moderator:
COL Nancy Jaax, Diplomate, ACVP
Pathology Division
U.S. Army Medical Research Institute of Infectious Disease
Ft. Detrick, Frederick, MD 21702-5011
NOTE: Click on images for larger views. Use
browser's "Back" button to return to this page.
Return to WSC Case Menu
Case I - 9735768 (AFIP 2639843)
-
- Signalment: Eleven-year-old, male, domestic shorthair,
feline.
-
- History: One large focal mass was noticed within the
skin and underlying subcutaneous tissues of the caudal dorsal
midline by the owner. An excisional biopsy was performed by the
referring veterinarian.
-
- Gross Pathology: One large focal tan mass, approximately
2.5 cm in diameter, was submitted for histologic examination.
-
- Laboratory Results: Cultures of the mass were positive
for Blastomyces dermatitidis.
-
- Contributor's Diagnosis and Comments: Severe focal
ulcerative pyogranulomatous mycotic dermatitis, cellulitis, and
myositis (blastomycosis), caudal dorsal midline.
-
- Etiology: Blastomyces dermatitidis.
-
- The histologic appearance of the section of skin and underlying
tissues is characterized by severe epidermal ulceration and large
accumulations of neutrophils intermixed with macrophages, plasma
cells, lymphocytes and epithelioid cells within the dermal and
subcutaneous tissues which extend into the underlying skeletal
musculature. Numerous spherical to slightly ovoid, thick, double
contoured walled organisms are also evident within the inflammatory
cell accumulations. Occasional organisms displaying broad based,
single budding are noted in some sections. Large, multifocal
to coalescing areas of necrosis are also evident scattered throughout
the tissues. The lesions extend to the edges of many of the sections.
-
- Blastomycosis is primarily a disease of humans and dogs but
can be seen in other animals, including the cat and horse. Blastomyces
dermatitidis, the causative agent of North American blastomycosis,
is a dimorphic fungus which produces a mycelial growth at room
temperature and yeast-like forms in tissue and culture at 37
degrees Celsius. The organism reproduces by budding and can be
found free or within macrophages in affected tissues. Although
the lung is considered the most common site of primary involvement,
primary cutaneous infections can also occur. The pulmonary form
of the disease has a chronic course characterized by exercise
intolerance and coughing. Systemic dissemination can occur with
lesions found within the lymph nodes, skin, eyes, central nervous
system, subcutaneous tissues, bones, joints, urogenital system
and other organs. Cutaneous lesions begin as small papules and
progressively develop into granulomas or pyogranulomas.
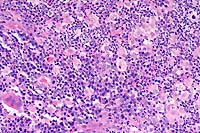 20x
obj.
20x
obj. 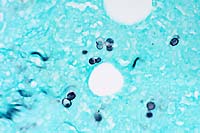 GMS
40x obj.
GMS
40x obj.
- Case 3-1. Haired skin. Diffusely (here, Left, 20x
obj) replacing normal dermal elements, there is an cellular infiltrate
composed of high numbers of neutrophils, fewer epithelioid macrophages,
lymphocytes, and plasma cells with rare foreign body type giant
cells (lower left). There are rare 15-20u diameter oval yeast
bodies with a basophilic central zone surrounded by a clear eccentric
halo (upper right). Gomori Methenamine Silver (Right, GMS 40x
obj) staining highlights yeast bodies which are occasionally
forming broad based buds.
-
- AFIP Diagnosis: Haired skin and subcutis: Dermatitis
and panniculitis, pyogranulomatous, diffuse, severe, with ulceration,
acanthosis, furunculosis, and yeast-like organisms, Domestic
Shorthair, feline, etiology consistent with Blastomyces dermatitidis.
-
- Conference Note: Blastomycosis is usually acquired
by inhalation of spores from the environment. The organism establishes
a primary infection in the lung, and becomes disseminated most
commonly to the lymph nodes, skin, eyes, bone, subcutaneous tissues,
external nares, brain and testes via the vascular and lymphatic
system. Less commonly, dissemination may occur to nasal passages,
mouth, prostate, liver, mammary gland, vulva, and heart. Occasionally,
primary cutaneous infection may occur from a puncture wound in
the skin. Such skin lesions begin as papules and develop into
abscesses. As the abscesses expand, the center undergoes cicatrization.
Because skin lesions more commonly result from disseminated infection,
cutaneous blastomycosis should arouse suspicion of systemic disease.
Lung lesions sometimes resolve by the time the sites of disseminated
infection become apparent.
-
- Soil is thought to be the reservoir for B. dermatitidis.
Four key environmental factors have been epidemiologically associated
with infection. These are moisture; sandy, acidic soil with organic
debris; disruption of the soil; and the presence of wildlife.
Most cases of blastomycosis occur along waterways. Many infections
are associated with sandy, acidic soil and organic debris, and
the disruption of soil may be caused by earth moving equipment
or landscaping. The presence of wildlife, particularly beavers
and waterfowl and their excreta, is also believed to play a part
in the occurrence of disease. In these environments, the organism
grows as a saprophytic mycelial form that produces infective
spores. At body temperatures, the organism transforms from the
mycelial form to the yeast form under the control of its bys-1
gene.
-
- As noted by the contributor, blastomycosis is most common
in dogs and people, but cats, horses, sea lions, wolves, ferrets,
dolphins and polar bears have also developed the disease. In
cats, pulmonary, cutaneous, and systemic forms occur. There does
not seem to be breed, age, or sex predisposition. In dogs, however,
sex and breed predispositions have been noted. Male dogs are
more frequently infected than females, and a greater percentage
of females with equally severe disease survive treatment. Sporting
dogs and hounds are at greater risk, probably due to more frequent
outdoor activity than other breeds. In general, young (one to
five-year-old), male, large breed dogs are most commonly infected.
In the southeast United States, occurrence is not seasonal, but
in other areas of the U.S., most cases occur from the late spring
through late autumn.
-
- A differential diagnosis that included cryptococcosis, histoplasmosis,
aspergillosis, coccidiomycosis, African histoplasmosis and South
American blastomycosis was considered by the conference attendees.
In tissue, Blastomyces dermatitidis occurs as 8-15 micron diameter,
spherical to oval, multinucleate, yeast-like cells, with thick,
doubly contoured, refractile walls and single, broad-based buds.
Coccidiodes immitis is larger, and reproduces by endosporulation
rather than budding. Histoplasma capsulatum is much smaller than
Blastomyces, and has narrow-based budding. Histoplasma capsulatum
var. duboisii (African histoplasmosis) may be confused with Blastomyces
dermatitidis, but the former has narrow-based buds and do not
contain multiple nuclei. Cryptococcus neoformans is characterized
by a wide, carminophilic capsule. Aspergillus organisms often
occur as radiating hyphae that branch dichotomously at acute
angles. Paracoccidiodes braziliensis, the cause of South American
blastomycosis, reproduces in tissue by multiple budding.
-
- Contributor: Animal Diagnostic Laboratory, Pennsylvania
State University, University Park, PA 16802.
-
- References:
- 1. Cote E, Barr SC, Allen C, Eaglefeather E: Blastomycosis
in six dogs in New York
state. J Amer Vet Med Assoc 210(4):502-504, 1997.
- 2. Jubb KVF, Kennedy PC, Palmer N: The respiratory system.
In: Pathology of
Domestic Animals, 4th ed., vol. 2, pp. 667, Academic Press, 1993.
- 3. Carter GR, Cole Jr. JR: In: Diagnostic Procedures in Veterinary
Bacteriology and Mycology. 5th ed., pp. 442-446, Academic Press,
1990.
- 4. Breider MA, Walker TL, Legendre AM, van Ee RT: Blastomycosis
in cats: five cases (1979-1986). J Amer Vet Med Assoc 193(5):570-572,
1988.
- 5. Nasisse MP, van Ee RT, Wright B: Ocular changes in a cat
with disseminated blastomycosis. J Amer Vet Med Assoc 187(6):629-631,
1985.
- 6. Legendre AM: Blastomycosis. In: Infectious Diseases of
the Dog and Cat, 2nd ed., pp. 371-377, WB Saunders Co., 1998.
- 7. Jones TC, Hunt RD, King NW: Diseases caused by fungi.
In: Veterinary Pathology, 6th ed., pp. 505-547, Williams and
Wilkins, 1997.
-
- International Veterinary Pathology Slide Bank:
Laser disc frame #'s 12365-12367.
-
Case II - A96359034 (AFIP 2639019)
-
- Signalment: Two-week-old, female Labrador Retriever.
-
- History: Two puppies from a litter of seven became
acutely ill with diarrhea ranging from bloody to mucoid. In one
of the puppies, there was severe abdominal cramping.
-
- Gross Pathology: Acute pulmonary congestion and edema.
Ileal serosal petechiation.
Laboratory Results:
Bacterial cultures:
1. Bitch's milk: Staphylococcus intermedius.
2. Puppy lung & intestine: E. coli.
-
- Virology: Electron microscopy of puppy intestinal
contents positive for parvovirus.
Contributor's Diagnosis and Comments: Enteritis, acute,
necrotizing with enterocyte intranuclear inclusions. Etiology:
Minute virus of canines (parvovirus).
Additional lesions present in the puppy were multifocal hepatic
necrosis and interstitial pneumonia with intravascular colonies
of small gram-negative bacilli suggesting an acute superimposed
bacteremia. Lymphoid necrosis in Peyer's patches and mesenteric
lymph nodes was attributed to the viral infection.
Two distinct parvoviruses are known to infect dogs. Canine parvovirus-type
1 (minute virus of canines) infection seems to be fairly widespread,
but clinical disease is uncommon. Canine parvovirus-type 2 infection,
first recognized in 1978, is also widespread, and infection commonly
results in severe disease with high mortality. The viruses differ
in antigenicity and tissue tropism.
Clinical disease due to CPV-1 can result from in utero infection
early in gestation with embryonic/fetal death. In utero infection
late in gestation results in the birth of normal puppies. Infection
in puppies generally less than three weeks of age can result
in clinical disease, but factors relating to clinical disease
versus inapparent infection are unknown. Clinically, the disease
is manifest as vomiting, diarrhea, crying, and dyspnea. Histologically,
the lesions affect primarily small intestine and lymphoid tissues.
In the small intestine, intranuclear inclusion bodies occur at
or near the villous tips with sloughing but minimal necrosis
of epithelial cells. Hyperplasia of crypt and villous epithelium
may also be noted. Necrosis and/or depletion of lymphocytes occur
in lymphoid tissues. According to one report, the Walter Reed
canine cell line is the only one that supports replication of
the CPV-1.
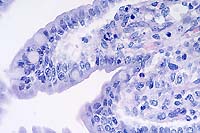 40x
obj.
40x
obj.
- Case 3-2. Small intestine. Demonstrates villous fusion,
swelling of mucosal epithelial cells, and multiple brick-shaped
amphophilic intranuclear inclusions.
 20x
obj.
20x
obj.
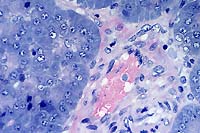
- Case 3-2. Lymph node. There is lymphoid depletion
of the paracortex, loss of follicular definition, and scattered
karyorrhexis of lymphoid cell nuclei (necrosis).
 40x
obj.
40x
obj.
- Case 3-2. Pancreas. Multifocally within a blood vessel
there are clusters of small bacilli.
-
- AFIP Diagnosis:
- 1. Small intestine: Enteritis, subacute, diffuse, mild, with
multifocal villar fusion, multifocal epithelial necrosis, and
numerous, villar tip, epithelial intranuclear inclusions, Labrador
Retriever, canine.
- 2. Lymph node; Peyer's patches: Lymphoid necrosis, diffuse.
- 3. Pancreas: Bacilli, intravascular and multifocal.
-
- Conference Note: Canine parvoviruses are small (20
nm diameter), nonenveloped, single-stranded DNA viruses that
require rapidly dividing cells, such as bone marrow cells, intestinal
epithelium, and lymphoid cells, for replication. Parvoviruses
are extremely stable and resistant to both adverse environmental
conditions and most common detergents and disinfectants, with
the exception of sodium hypochlorite (household bleach).
-
- The domestic dog is the only proven host for CPV-1, although
other canids are probably susceptible. Serologic evidence suggests
that it has a widespread distribution in the dog population,
but clinical disease is generally restricted to pups less than
three weeks old. Natural disease develops in these young pups
through oronasal exposure or in utero infection and causes enteritis,
mild pneumonia, and occasional myocarditis or sudden death. Because
of the limited number of reported cases, the clinical importance
of CPV-1 infection is not completely known.
Histologically, the intestinal lesions produced by CPV-1 infection
are remarkably different from those of CPV-2 enteritis. CPV-2
infection results in loss of normal villar architecture, collapse
of the mucosa, extensive blunting and fusion of villi, and often
severe crypt epithelial necrosis. Epithelial intranuclear inclusions
are uncommonly observed. In contrast, relatively normal villar
architecture is maintained in CPV-1 infection, and there is crypt
epithelial hyperplasia. Villar epithelial cells are often vacuolated
and contain numerous intranuclear inclusions, and there may be
a mild inflammatory infiltrate.
-
- Several other histologic lesions may occur in parvoviral
infections. Varying degrees of lymphoid necrosis and depletion,
both within Peyer's patches and lymph nodes, are described in
both types of parvovirus infection. Interstitial pneumonia, bronchitis,
pneumonitis, and the presence of intranuclear inclusions within
the bronchiolar epithelium can occur with CPV-1, and are especially
associated with experimental infections. Infrequently, a nonsuppurative
myocarditis may occur in CPV-1 infection, and varying histologic
lesions have been described including interstitial edema, infiltration
by mononuclear inflammatory cells, myocardial necrosis and mineralization,
and occasionally the presence of intranuclear inclusions within
myocardiocytes.
-
- Gross lesions in infected puppies are often mild. In experimental
infections, lesions noted included red-gray consolidation of
ventral and hilar areas of the apical and cardiac lobes of the
lung, and bronchial and mediastinal lymphadenopathy. Lesions
in natural infections vary and include enlarged lymph nodes,
streaking of the myocardium, atelectatic lungs, and soft, pasty
stools in the intestinal tract. The disease may cause stillbirths
or the birth of weak pups in infected bitches.
-
- Minute virus of canines may cause spontaneous disease in
young pups. Failure to subject stillborn or neonatal pups to
pathologic study may be the cause for the lack of reports. Pneumonia
and enteritis, and occasionally myocarditis, characterize the
pathological finidings in CPV-1 infection. The virus may provoke
only mild or vague signs, and fatal cases may be diagnosed as
"fading pups". Minute virus of canines should be considered
in the differential diagnosis for pups that die when less than
three weeks of age and in cases of failure to conceive or fetal
death.
References:
- 1. Barker IK, van Dreumel AA, Palmer N: The alimentary system.
In: Pathology of Domestic Animals, 4th ed., Jubb, Kennedy, Palmer
eds., vol. 2, pp. 141-199, Academic Press, 1993.
- 2. Carmichael LE, Schlafer DH, Hashimoto A: Pathogenicity
of minute virus of canines (MVC) for the canine fetus. Cornell
Vet 81:151-171, 1991.
- 3. Harrison LR, Styer EL, Pursell AR, Carmichael LE, Nietfeld
JC: Fatal disease in nursing puppies associated with minute virus
of canines. J Vet Diag Invest 4:19-22, 1992.
- 4. Macartney L, et. al.: Characterization of minute virus
of canines (MVC) and its pathogenicity for pups. Cornell Vet
78:131-145, 1988.
- 5. Hoskins JD: Canine viral enteritis. In: Infectious Diseases
of the Dog and Cat, 2nd ed., pp. 40-46, WB Saunders Co., 1998.
-
- Contributor: Texas Veterinary Medical Diagnostic Lab,
P.O. Box 3200, Amarillo, Texas 79116-3200.
-
-
Case III - D96 3803 (AFIP 2642051 [corrected])
-
- Signalment: Eleven-year-old, male, neutered Domestic
Longhaired cat.
-
- History: This cat had fever of 105.8 and did not respond
to enrofloxacin or dexamethasone. He then became lethargic, shocky
and died.
-
- Gross Pathology: None described.
Laboratory Results: None.
Contributor's Diagnosis and Comments: Splenitis, histiocytic,
diffuse, severe, with numerous intracellular protozoal organisms,
characteristic of Cytauxzoon.
-
- Etiology: Cytauxzoon felis.
There are many intravascular and extravascular macrophages in
the spleen filled with schizonts characteristic of Cytauxzoon
felis infection. Cytauxzoon felis is classified in the family
Theileriidae. It is transmitted by ixodid ticks and is not contagious
by direct contact exposure. The bobcat is a natural host. Cats
infected with Cytauxzoon felis develop signs of anemia, icterus,
pyrexia, and depression. Early diagnosis of this disease can
be achieved by examination of peripheral blood for erythroparasitemia.
Cytauxzoon felis trophozoites are ring-forms and present within
the cytoplasm of erythrocytes. This differs from feline infectious
anemia caused by Hemobartonella felis in which the organisms
are coccoid and present on the surface of red blood cells. Cytauxzoonosis
is fatal in cats, and so far there is no drug available for the
treatment of this disease.
-
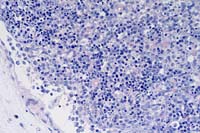
 10x
obj.
10x
obj.
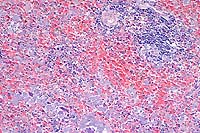
- Case 3-3. Spleen. There is little white pulp, and
here it is predominantly around a small arteriole. Multifocally
within the red pulp and vascular sinuses, there are abundant
ill defined large granular basophilic cells (interpreted as macrophages).
 20x
obj.
20x
obj.
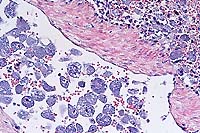
- Case 3-3. Spleenic artery. Within the splenic arteries
there are abundant large 20-30u cells with granular cytoplasm,
open faced nuclei and a single prominant nuclei (interpreted
as macrophages containing protozoal organisms). Similar macrophages
are scattered around the artery in the red pulp.
-
- AFIP Diagnosis: Spleen: Histiocytosis, intravascular
and diffuse, moderate, with intrahistiocytic protozoal schizonts,
Domestic Longhair, feline, etiology consistent with Cytauxzoon
felis.
-
- Conference Note: Most conference participants preferred
the diagnosis of splenic histiocytosis over splenitis based upon
the lack of histological changes associated with inflammation.
The lesion is characterized by an infiltrate composed almost
entirely of histiocytes that are filled with basophilic organisms
(schizonts), but there is lack of other inflammatory cells and
vascular changes that normally characterize an inflammatory process.
Cytauxzoon is classified in the order Piroplasmida and family
Theileriidae. This family has both an erythrocytic and a tissue
(leukocytic) phase. Large schizonts of C. felis develop in macrophages,
whereas in Theileria the exoerythrocytic stage occurs primarily
within lymphocytes. The Babesiidae, a related family, is characterized
by having a primarily erythrocytic phase in the mammalian host,
and its morphological features are indistinguishable from the
erythrocytic form of Cytauxzoon. Cytauxzoon felis, B. equi, and
B. rodhaini have been linked to both the babesias and theilerias
by RNA gene sequence analysis, and it has been suggested that
these organisms be reclassified within a separate family.
Ticks are implicated as the natural vector for Cytauxzoon, because
most cases of infection have been associated with the presence
of these parasites on the hosts. Experimentally, Dermacentor
variabilis can transmit the organism from bobcats to domestic
cats. In a white tiger that developed a natural, fatal infection
in Florida, two female Lone Star ticks (Amblyomma americanum)
were present on the inguinal skin. In the life cycle of C. felis,
schizonts develop within mononuclear phagocytes, initially as
indistinct vesicular structures and later as large, distinct
nucleated schizonts that actively undergo division by true schizogony
and binary fission. Later in the course of the disease, schizonts
develop buds (merozoites) that separate and eventually fill the
entire host cell. The host cell probably ruptures, releasing
merozoites into the tissue fluid and blood. Merozoites are then
believed to enter erythrocytes to form the intraerythrocytic
stage. Merozoites appear in macrophages one to three days before
they are observed in erythrocytes.
-
- Clinically, the disease in cats is characterized by fever,
depression, dyspnea, anorexia, lymphadenopathy, anemia, and icterus
leading to death in three to six days. Gross findings include
pale or icteric mucous membranes, petechiae and ecchymoses in
the lung, heart, lymph nodes and on mucous membranes, splenomegaly,
lymphadenomegaly, and hydropericardium. Microscopically, numerous
large schizonts are present within the cytoplasm of endothelial-associated
macrophages. Infected macrophages become markedly enlarged (up
to 75 micrometers) and may occlude the lumens of numerous vessels
of many tissues, especially the lungs. Minimal inflammatory reaction
is present in tissues.
-
- Each schizont may contain numerous merozoites. Ultrastructurally,
schizonts lack a parasitophorous vacuole, and individual merozoites
possess rhoptries. Merozoites within erythrocytes, best seen
on peripheral blood or tissue impressions, are variable in morphology
and can occur as round, oval, or signet ring-shaped bodies 1-5
micrometers in diameter with a small, peripherally placed basophilic
nucleus.
-
- Organisms that must be distinguished from the intraerythrocytic
phase of C. felis include Babesia and Hemobartonella, because
the blood stage may appear similar to the ring forms of Hemobartonella
and to the piriforms of Babesia. Unlike Cytauxzoon, however,
babesiosis and hemobartonellosis do not have a tissue stage of
infection. Differential diagnosis for the tissue phase of cytauxzoonosis
includes other small (less than 5 m), intrahistiocytic organisms
such as Toxoplasma, Leishmania, and Histoplasma.
-
- References:
- 1. Cowell RL, Panciera RJ, Fox JC, Tyler RD: Feline cytauxzoonosis.
Compend Cont Ed Pract Vet 10:731-735, 1988.
- 2. Garner MM, Lung NP, Citino S, Greiner EC, Harvey JW, Homer
BL: Fatal cytauxzoonosis in a captive-reared white tiger (Panthera
tigris). Vet Pathol 33:82-86, 1996.
- 3. Kier AB, Greene CE: Cytauxzoonosis. In: Infectious Diseases
of the Dog and Cat, 2nd ed., pp. 470-473, WB Saunders Co., 1998.
- 4. Jones TC, Hunt RD, King NW: Diseases due to protozoa.
In: Veterinary Pathology, 6th ed., pp. 599-600, Williams and
Wilkins, 1997.
- Contributor: PAL-PATH, Inc., 1277 Record Crossing Road, Dallas,
TX 75235.
-
- International Veterinary Pathology Slide Bank:
Laser disc frame #'s 5298-5300, 5829-5831, 7986, 14300, 9642.
-
Case IV - 97-26234 (AFIP 2642418 [corrected])
-
- Signalment: Four-year-old, female, spayed, Pug, canine.
-
- History: The dog was originally presented for peripheral
lymphadenopathy which was diagnosed via excisional lymph node
biopsy as granulomatous lymphadenitis secondary to mycobacteriosis.
Lymph node culture revealed Mycobacterium avium. The dog did
well for two years on a multiantibiotic treatment regimen. Regularly
scheduled appointments to monitor the disease included serum
chemistries and palpation of peripheral lymph nodes which initially
regressed in size. Two years after diagnosis, the lymph nodes
began to enlarge again, and since the dog was shedding M. avium
in the feces as confirmed by culture, she was euthanized at that
time.
- Gross Pathology: The spleen was greatly enlarged (2800
g; 30x6x4 cm). It was meaty in texture and was mottled cream-colored
to yellow throughout the parenchyma. The mesenteric and cecocolic
lymph nodes were enlarged and uniformly cream-colored to tan
on cut section.
-
- Laboratory Results: Blood chemistries: Hypoalbuminemia,
hypocholesterolemia, decreased BUN.
-
- Contributor's Diagnosis and Comments: Disseminated
granulomatous disease, mycobacteriosis, Mycobacterium avium.
-
- Although gross changes were confined to the spleen and intestinal
lymph nodes, there were multifocal infiltrates of macrophages
in the lungs, liver, kidneys, thyroid gland, bone marrow, intestines,
and other lymph nodes. Acid-fast stains of sections revealed
numerous acid-fast bacteria in macrophages in all tissues examined.
Splenic architecture was effaced by sheets of macrophages with
binucleate and multinucleate forms and epithelioid macrophages
with a few foci of residual lymphoid cells. The bone marrow was
almost completely effaced by macrophages, although some myeloid
and erythroid cells remained. Perivascular infiltrates of macrophages
were present throughout the lungs and liver and in the lamina
propria of multiple intestinal sections.
-
- Mycobacteriosis in dogs is uncommon in the U.S. since the
effort to eradicate tuberculosis in domestic animals has been
largely successful. The dog is experimentally equally susceptible
to Mycobacterium tuberculosis and M. bovis, but is considered
resistant to M. avium. Infection is generally via inhalation
of aerosols or ingestion of infected material. The size of the
inoculum, the number of times an animal is exposed to the organism,
and the immune status of the individual all determine whether
an active mycobacterial infection becomes established.
-
- While a route of exposure to M. avium was not determined
for this dog, a possibility that remains is that the dog frequently
visited a location that was adjacent to a major waterfowl staging
area. The dog had a habit of being a "garbage hound"
and would always ingest things it found on the ground or floor.
If this dog was ingesting M. avium-infected waterfowl droppings
or inhaling infected aerosols repeatedly, the cumulative exposure
to M. avium could have been great enough for infection to occur.
There was no indication given in the record regarding the immune
status of this dog.
 40x
obj.
40x
obj.
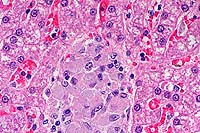
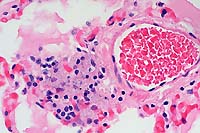
- Case 3-4. Liver. Demonstrates focus of epithelioid
macrophages replacing normal hepatic cords. These cells are filled
with ill defined rod shaped structures.
 40x
obj.
40x
obj.
- Case 3-4. Lung. Similar clusters of epithelioid macrophages
and lymphocytes expand alveolar septa near moderate sized blood
vessels. Macrophages contain granular to rod shaped material.
 40x
obj. Zeil-Neilson
40x
obj. Zeil-Neilson
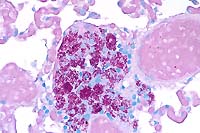
- Case 3-4. Lung. Cell clusters adjacent to vessels
like those described above contain myriad acid fast bacilli.
-
- AFIP Diagnosis:
- 1. Liver: Hepatitis, granulomatous, portal, central, and
multifocal, moderate, with numerous intrahistiocytic bacilli.
- 2. Lung: Pneumonia, granulomatous, perivascular, multifocal,
moderate, with numerous intrahistiocytic bacilli.
- 3. Lung: Congestion, diffuse, moderate, with abundant alveolar
edema.
-
- Conference Note: Mycobacterial infections in man and
animals are caused by bacteria belonging to the family Mycobacteriaceae,
order Actinomycetales. Mycobacterium is a genus compromising
morphologically similar, aerobic, gram- positive, non-spore forming,
and non-motile bacilli with wide variations in host affinity.
They have the unique property of being acid-fast due to the high
lipid content of mycolic acid in the cell wall.
-
- The bacteria have been subdivided into several groups and
individual species based on biochemical and culture characteristics.
The species causing "classic" tuberculosis are termed
the M. tuberculosis complex (MTC) and include M. bovis, M. tuberculosis,
M. africanum (rare cause of human TB in Africa), and M. microti
(a rodent pathogen that has been reported to infect cats). Those
species grouped together causing the syndrome of M. avium complex
(MAC), sometimes referred to as "avian mycobacteriosis",
include Mycobacterium avium-intracellulare and M. avium susp.
paratuberculosis. The latter, which is the cause of Johne's disease
in ruminants (ruminant paratuberculosis), can infect monogastric
animals and produces lesions in stump-tailed macaques that are
very similar to Crohn's disease in man, thus implicating this
bacteria as a potential etiology for the human disease. Another
separate group of myocobacterial infections is caused by M. leprae
and called either leprosy or Hansen's disease, while feline and
murine leprosy is caused by M. lepraemurium. The final group,
termed "atypical mycobacteriosis", can be described
as the localized opportunistic skin and subcutaneous infections
caused by saprophytic and rapidly growing mycobacteria, e.g.
M. fortuitum, M. chelonae, etc.
-
- Classic tuberculosis in immunocompetent humans results in
the formation lumps or nodules called tubercles (from the Latin
word "tuberculum"), and histologically consists of
well-formed granulomas composed of epithelioid macrophages, Langhans-type
multinucleate macrophages, and lymphocytes. Acid-fast mycobacteria
are few and difficult to find. Granuloma formation, which fundamentally
requires sufficient numbers of functioning macrophages and T
helper-1 lymphocytes, is often absent in humans and nonhuman
primates that are immunocompromised due to concurrent infection
with immunodeficiency viruses.
-
- Instead, these individuals and animals develop disseminated
disease with diffuse granulomatous inflammatory infiltrates and
contain more abundant acid-fast organisms.
- In dogs and cats, MAC infections are uncommon; in humans
MAC organisms are of low virulence in immunocompetent individuals,
but cause infections in 15 to 24% of patients with HIV infections
that become severely immunocompromised (less than 60 CD4+ cells
per cubic mm). Most infections in all three species are caused
by M. avium-intracellulare, often originate in the gastrointestinal
tract via oral ingestion, and become widely disseminated to the
liver, spleen, lung, and lymph nodes. Spread to skin, bone, cervical
vertebrae, mammary gland, and serous surfaces may also occur
in animals.
-
- Histologically, MAC infections in most mammals, including
humans, are characterized by a diffuse granulomatous, inflammatory
reaction containing large numbers of epithelioid macrophages
without necrosis, fibrosis, or calcification. Macrophages are
packed with high numbers of acid-fast bacilli, which may appear
as "negative images" in hematoxylin and eosin stained
sections. Langhans-type multinucleated macrophages may be present,
but not always. There is little lymphocytic response. In dogs
and cats, lesions are multifocal and coalescing to diffuse, and
the inflammatory cells often displace or replace affected tissues
creating a "sarcomatous" appearance.
-
- The histological similarities of MAC infections in cats and
dogs to immunocompromised humans infected with MAC suggest that
innate immunodeficiency may predispose these small animals to
the disease. Histologically, the character of the inflammation
in immunodeficient humans concurrently infected with classic
tuberculosis more closely resembles MAC infections in dogs and
cats than the character of classic tuberculosis in immunocompetent
individuals, further supporting the theory of a deficiency in
cell-mediated immunity in these animals. Finally, there is evidence
that a genetic predisposition to MAC infections may exist in
basset hounds, miniature schnauzers, and Siamese cats.
-
- References:
- 1. Feldman WH: The pathogenicity for dogs of bacilli of avian
tuberculosis. J Am Vet Med Assoc 76:399-419, 1930.
- 2. Francis J: Tuberculosis in small animals. Mod Vet Pract
39-42, 1961.
- 3. Snider WR: Tuberculosis in canine and feline populations.
Am Rev Resp Dis 104:877-887, 1971.
- 4. Liu S: Canine tuberculosis. J Am Vet Med Assoc 177:164-167,
1980.
- 5. Thoen C O, Himes E M: Mycobacterium. In: Pathogenesis
of Bacterial Infections in Animals, pp. 26-37, Iowa State University
Press, 1986.
- 6. Carpenter J L, et al.: Tuberculosis in five Bassett Hounds.
J Am Vet Med Assoc 192:1563-1568, 1988.
- 7. Shackelford CC, Reed WM: Disseminated Mycobacterium avium
infection in a dog. J Vet Diagn Invest 1:273-275, 1989.
- 8. Clercs C, et al.: Tuberculosis in dogs: A case report
and review of the literature. J Am Anim Hosp Assoc 28:207-211,
1992.
- 9. Eggers JS, et al.: Disseminated Mycobacterium avium infection
in three miniature schnauzer litter mates. J Vet Diagn Invest
9:424-427, 1997.
-
- Contributor: University of Minnesota, Veterinary Diagnostic
Laboratory, College of Veterinary Medicine, 1333 Gortner Avenue,
St. Paul, MN 55108.
-
- International Veterinary Pathology Slide Bank:
Laser disc frame #'s 21897, 9127, 9926.
-
- Ed Stevens, DVM
Captain, United States Army
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: STEVENSE@afip.osd.mil
-
- * The American Veterinary Medical Association and the American
College of Veterinary Pathologists are co-sponsors of the Registry
of Veterinary Pathology. The C.L. Davis Foundation also provides
substantial support for the Registry.
-
Return to WSC Case Menu
 20x
obj.
20x
obj.  GMS
40x obj.
GMS
40x obj.
 20x
obj.
20x
obj.  GMS
40x obj.
GMS
40x obj.
 40x
obj.
40x
obj.
 20x
obj.
20x
obj.
 40x
obj.
40x
obj.
 10x
obj.
10x
obj.
 20x
obj.
20x
obj.
 40x
obj.
40x
obj.
 40x
obj.
40x
obj.
 40x
obj. Zeil-Neilson
40x
obj. Zeil-Neilson