Results
AFIP Wednesday Slide Conference - No. 8
12 November 1997
Conference Moderator: Dr. R. Keith Harris
Diplomate, ACVP
Product Safety Assessment, Searle
4901 Searle Parkway
Skokie, IL 60077
Return to WSC Case Menu.
Case I - 97-17535 (AFIP 2596340)
Signalment: 13-month-old, castrated, male American quarter
horse
History: The yearling was submitted for euthanasia after
skin lesions located on the distal limbs failed to respond to
treatment with topical antibacterial agents and systemic antibiotics.
The skin lesions developed two weeks after the yearling had an
upper respiratory infection. The specific cause of the respiratory
infection was not specified.
Gross Pathology: Large areas of skin necrosis and sloughing
with exposure of bone were present on all distal limbs. Exudation
and hemorrhages were also noted. Areas of indurated skin were
located adjacent to the areas of skin necrosis.
Laboratory Results: Staphylococcus aureus and Streptococcus
zooepidemicus were isolated by routine aerobic skin cultures.
Sections of skin were positive for equine IgG and negative for
IgM. The fluorescence was widespread.
- Contributor's Diagnosis and Comments: Subacute severe
cutaneous vasculitis.
The history, clinical signs and histologic findings are consistent
with equine purpura hemorrhagica. This syndrome has been associated
with a variety of respiratory diseases. No lesions were detected
at necropsy in the respiratory tract, mucous membranes or lymph
nodes. The syndrome usually occurs in horses over two-years-old.
-
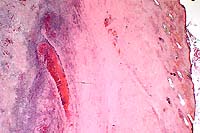
- Case 8-1. Skin. Infarct contains a dermal vessel with
a necrotic wall and a thrombus composed of RBC's, neutrophils,
and fibrin. Numerous neutrophils infiltrate the vessel wall and
extend in the surrounding connective tissue.
- AFIP Diagnosis: Haired skin: Dermatitis and cellulitis,
suppurative, chronic, diffuse, severe, with leukocytoclastic
vasculitis and focally extensive necrosis (infarct), American
quarter horse, equine.
Conference Note: Gram stains performed at the AFIP demonstrated
numerous gram-positive cocci within the infarcted area; some are
present within the lumina of blood vessels. Because of the presence
of the bacteria and the suppurative reaction, bacterial infection,
particularly septicemia, is included in the differential diagnosis.
Cutaneous vasculitis in horses is usually seen as a secondary
manifestation of a variety of conditions, including infectious,
toxic, neoplastic, and immunologic disorders. Cutaneous arteritis
has been reported in a horse with granulomatous enteritis3. Most
recognized syndromes of vasculitis in horses have characteristics
similar to those of hypersensitivity-vasculitis seen in humans1,
in which the most common inflammatory pattern is neutrophilic
infiltration of venules in the dermis and subcutis. Usually there
is leukocytoclasis, or neutrophil fragmentation with nuclear debris
in and around vessels, and fibrinoid necrosis of vessel walls.
Purpura hemorrhagica is the most commonly diagnosed cutaneous
vasculitic syndrome in horses, and it has also been reported in
dogs and pigs. This condition is thought to be caused by a type
III (immune complex) hypersensitivity reaction following Streptococcus
equi or other respiratory system infections. Extensive petechiation
and ecchymosis are seen in the skin and mucous membranes; in horses,
there is often localized or generalized edema.
Other infectious diseases of horses in which cutaneous vasculitis
is a common feature include equine viral arteritis, equine infectious
anemia, and equine ehrlichiosis.
Contributor: Veterinary Diagnostic and Investigational Laboratory,
University of Georgia, College of Veterinary Medicine, P.O. Box
1389, Tifton, GA 31793
References:
- 1. Morris DD: Cutaneous vasculitis in horses: 19 cases (1978-1985).
JAVMA 191:460-464, 1987.
- 2. Valli VEO: The hematopoietic system. In: Pathology of
Domestic Animals, Jubb KVF, Kennedy PC, and Palmer N, eds., Academic
Press, 4th edition, vol 3, p. 262, 1993.
- 3. Woods PR, Helman RG, Schmitz DG: Granulomatous enteritis
and cutaneous arteritis in a horse. Journal of the American Veterinary
Medical Association 203(11):1573-1575, 1993.
International Veterinary Pathology Slide Bank:
Laser disc frame #706, 4934, 7658, 15700-06, 15724, 15769.
Case II - 96120352 (AFIP 2596315)
Signalment: Adult, male, mixed breed, 25 kg, canine.
History: Two of the owner's dogs were seen vomiting
raw meat on 1 December. One dog recovered and the other died.
This dog showed signs of depression, lethargy and vomiting on
3 December and was presented to the Oklahoma State University
Veterinary Medical Teaching Hospital on 5 December with expiratory
dyspnea, dehydration, and depression. BUN, phosphate and creatinine
were increased. Radiographs revealed a pneumomediastinum. Arterial
blood gas showed decreased PO2. The dog died on 6 December.
Gross Pathology: Lesions were confined to the cardiopulmonary
system. There were numerous epicardial and endocardial hemorrhages
ranging from petechiae to 1.0 cm in diameter. Within the trachea
was a moderate amount of serosanguinous fluid. The lungs were
diffusely red-brown, firm and heavy. Cut surfaces exuded abundant
blood.
Laboratory Results: Toxicology: Paraquat was detected
in liver and kidney samples, but was not quantified. The vomitus
contained 567 ppm of paraquat.
Contributor's Diagnosis and Comments: Lung, pulmonary
edema and hemorrhage, severe, with concurrent fibrin exudation.
Cause: Acute paraquat toxicosis.
- Exposure to paraquat appeared malicious, whereby several
dogs were fed raw meat containing paraquat.
-
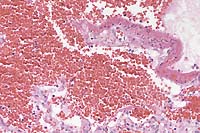
- Case 8-2. Lung. Diffuse hemorrhage in the alveoli
with focal fibrinoid necrosis of an alveolar septum. Other septae
have a mild increase in fibroblastic cells. 20X
-
- AFIP Diagnosis: Lung: Hemorrhage, diffuse, severe,
with multifocal septal necrosis and interstitial fibroplasia,
mixed breed, canine.
Conference Note: Paraquat is one of two widely used
dipyridyl broad- spectrum herbicides (the other being diquat).
Ingestion, inhalation, or dermal exposure to even small amounts
of either compound may result in death within 24 hours due to
necrosis of lung, liver, heart, kidneys, and adrenal glands.
Following a single large dose of paraquat by any route, pulmonary
edema and hemorrhage develop within hours and may lead to death.
Type I pneumocytes are the target cells in acute and chronic poisoning.
Paraquat is actively concentrated in these cells, and causes an
increase in both the consumption of O2 and the oxidation of NADPH
via NADPH-cytochrome P450 reductase. Resultant reactive oxygen
species are thought to play a key role in the acute lung injury.
Fibrosis develops as soon as 5 to 10 days after exposure to paraquat.
Following the initial pulmonary epithelial injury, there is intraalveolar
infiltration of profibroblasts through gaps in the epithelial
basement membranes. This is followed by connective tissue synthesis
on the luminal side of the epithelial basement membrane with differentiation
of interstitial cells into myofibroblasts and smooth muscle cells,
incorporation of areas of intraalveolar fibrosis into the interstitium,
and coalescence of alveolar walls. Alveolar macrophages contribute
to the marked fibrosis by releasing both fibronectin and fibroblast
growth factors after paraquat exposure.
Unlike paraquat, diquat intoxication results in intracerebral
hemorrhage and a high incidence of acute renal failure. Pulmonary
fibrosis is not a common finding with diquat poisoning.
Contributor: Department of Anatomy, Pathology, and Pharmacology,
250 Vet. Med. Bldg., Oklahoma State University, Stillwater, OK
74078-2007
References:
- 1. Nagata T, Kono I, Masaoka T, Akahori F: Acute toxicological
studies on paraquat: pathological findings in beagle dogs following
single subcutaneous injections. Vet Hum Toxicol 34:105-111, 1992.
- 2. Ali S, Jain SK, Abdulla M, and Athar M: Paraquat induced
DNA damage by reactive oxygen species. Biochem Mol Biol Int 1996
May;39(1):63-7.
- 3. Dungworth DL: The respiratory system. In: Pathology of
Domestic Animals, Jubb KVF, Kennedy PC, and Palmer N, eds., Academic
Press, 4th edition, vol 2, pp. 605-606, 1993.
International Veterinary Pathology Slide Bank:
Laser disc frame #3723, 6665-6, 6683, 8292-5, 15457, 15500, 15503-4,
15509.
Case III - 97A-22479 (AFIP 2592909)
Signalment: 15-week-old, male, Labrador Retriever.
History: This dog was presented for necropsy with a
history of respiratory difficulty and persistent vomiting prior
to death. Canine distemper was diagnosed in a litter-mate a week
earlier.
Gross Pathology: The animal was emaciated and the gastric
wall was markedly thickened with edema fluid. The mucosa was diffusely
hyperemic and slightly raised irregular gray foci of mucosal necrosis
were widely distributed over thickened gastric folds. Partially
digested blood was present in the small intestines.
Laboratory Results: Fluorescent antibody (FA) test for
canine distemper was positive on multiple tissues. FA tests for
canine parvovirus was negative. Serratia odorifera, Acinetobacter
sp., and Enterobacter sp. were isolated in heavy growth from the
gastric mucosa. Fungal cultures were negative. Tachyzoites in
tissue stained positively with an immunohistochemical stain for
Toxoplasma gondii.
Contributor's Diagnosis and Comments: Acute diffuse
necrotizing gastritis with intralesional tachyzoites (Toxoplasma
gondii and canine distemper virus infection).
The stomach is characterized by coalescing to diffuse mucosal
and submucosal necrosis with hemorrhage and marked submucosal
edema. Glandular epithelial cells are pyknotic or reduced to cell
debris. There is necrosis of vascular walls in the submucosa.
The lamina propria and submucosa are diffusely infiltrated with
neutrophils, macrophages, and fewer plasma cells. Gastric epithelial
cells and smooth muscle cells contain numerous tachyzoites within
the cytoplasm. Numerous extracellular tachyzoites are free within
the lamina propria and submucosa, and monocytes within blood vessels
contain tachyzoites. Tachyzoites associated with focal areas of
necrosis were also present in liver, pancreas, adrenal gland,
spleen, pancreas, lung, brain and myocardium
- Disseminated toxoplasmosis is a common secondary infection
in dogs that are immunosuppressed by canine distemper virus infection.
However, severe gastritis with gastric signs is not usually reported.
Increasingly, physicians are reporting similar gastritis due
to Toxoplasma gondii in AIDS patients as a presenting complaint.
Diagnosis can be made by a gastric biopsy. Both human and canine
cases of gastric toxoplasmosis represent recrudescence of latent
infections in immunocompromised hosts.
-
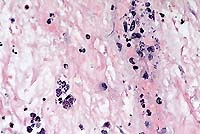
- Case 8-3. Stomach. Within a vessel wall in the submucosa,
there are many extracellular banana-shaped Toxoplasma gondii
zoites with scattered mixed leukocytes (vasculitis). An intracellular
protozoal cyst containing numerous zoites is also present (lower
left). 40X
- AFIP Diagnoses:
- 1. Stomach: Gastritis, transmural, necrotizing, acute, diffuse,
severe, with necrotizing vasculitis and intracellular and extracellular
protozoal zoites, Labrador Retriever, canine.
2. Lymph node: Lymphoid depletion, diffuse, severe, with multifocal
necrosis and sinus histiocytosis.
Conference Note: Sections of lymph node were not present
in all slides. Lymphoid depletion was attributed to the viral
infection.
In dogs naturally infected with canine distemper virus (CDV)
via aerosol exposure, viral replication occurs initially in macrophages
of the bronchial lymph nodes and tonsils. Within 2-5 days post-exposure,
the virus may be found in all lymphatic tissues, including bone
marrow, thymus and spleen. Necrosis of lymphoid elements leads
to prominent lymphoid depletion and an immunocompromised animal.
At this point, the dog becomes viremic and febrile. The infection
is primarily confined to lymphoid tissues for 6-9 days. Approximately
50% of infected dogs will develop a neutralizing antibody response
within 1-2 weeks post-exposure, and will clear the virus. In other
animals, the lymphatic infection persists and spreads to epithelial
tissues of the alimentary, respiratory, and urogenital tracts,
skin, endocrine glands, and possibly the CNS. These animals steadily
deteriorate, and usually die within 15-31 days post-exposure.
The disease is a summation of the effects of the virus and of
secondary infections, such as toxoplasmosis. Toxoplasmosis as
a clinical disease seldom occurs in dogs other than in association
with CDV or other immunosuppressive conditions.
Contributor: Department of Pathology, College of Veterinary
Medicine, University of Georgia, Athens, GA 30602
References:
- 1. Dubey JP, Green CE, Lappin MR: Toxoplasmosis and neosporosis.
In: Infectious Diseases of the Dog and Cat. CE Greene, ed. WB
Saunders Co., Philadelphia, 1990: pp. 818-834.
- 2. Alpert L, Miller M, Alpert E, et al: Gastric toxoplasmosis
in acquired immunodeficiency syndrome: antemortem diagnosis with
histopathologic characterization. Gastroenterology 110:258-264,
1996.
- 3. Dungworth DL: The respiratory system. In: Pathology of
Domestic Animals, Jubb KVF, Kennedy PC, and Palmer N, eds., Academic
Press, 4th edition, vol 2, pp. 617-624, 1993.
International Veterinary Pathology Slide Bank:
Laser disc frame #9099, 10444-6.
Case IV - 19882 (AFIP 2595302)
Signalment: 10-year-old, neutered, male, Domestic Shorthair
cat.
History: This animal had been missing from its home
in New York state for approximately one month. A small mass was
found in the subcutaneous tissue at the rostroventral surface
of the left mandible. The lower left canine tooth was missing,
and there was an accumulation of necrotic tissue within the vacant
dental alveolus. The patient had ptyalism and mucopurulent discharge
from the eyes and nose. Initial histopathological examination
revealed mild to moderate, chronic- active inflammation associated
with helminth fragments. Moderate fibrosis, with surgical artifact,
and accumulations of inflammatory cellular debris within the section,
prevented accurate taxonomic identification of the organism. Neoplastic
change was not identified in the initial tissue sections examined,
most likely due to the small size of the wedge biopsy. The owners
declined surgical excision of the mass. A fecal examination performed
after the surgical biopsy was negative for parasites and eggs.
The cat was re-examined 47 days after the initial examination
and surgical biopsy. Profuse ptyalism was again noted, and the
soft tissue adjacent to the right mandible was markedly swollen,
to the point that the animal was unable to close its mouth. The
site was painful upon palpation and the cat was extremely thin.
The owners elected euthanasia and permitted a limited necropsy.
Gross Pathology: Relevant necropsy findings included
swelling of soft tissues adjacent to both mandibles and mild swelling
at the base of the tongue. Multifocal, disseminated, 2-3 mm white
foci were distributed over the capsular surface of the liver.
The remainder of the necropsy findings were unremarkable. Sections
of mandible, facial skin, oral mucosa, tongue, diaphragm, and
liver were fixed in 10% neutral buffered formalin and submitted
for histopathological examination.
Histopathology: In the tissue sections from the oral
cavity, there was invasion of the submucosa and connective tissues
by a poorly encapsulated and densely cellular squamous cell carcinoma.
Neoplastic cells formed small nests and cords that invaded the
connective tissue, skeletal myofibers, and mandibular bone. The
cells were large and polygonal with intercellular bridges and
abundant well- demarcated eosinophilic cytoplasm. Nuclei were
large and round with a stippled chromatin pattern and contained
large, central, magenta nucleoli. There were 0-2 mitotic figures
per high power field, with occasional bizarre mitotic forms. Occasional
brightly eosinophilic keratin pearls were in the centers of the
largest accumulations of neoplastic cells. Also within the larger
foci of neoplastic cells, there were abundant cystic accumulations
of necrotic debris which were occasionally admixed with small
clusters of neutrophils. The mass was partly divided and surrounded
by moderately desmoplastic stroma. There were numerous small blood
vessels within the mass. Between the nests of cells there were
numerous necrotic myofibers that were hypereosinophilic, swollen,
and had lost cross-striations. At the periphery of the mass, necrotic
mandibular bone was invaded by the neoplastic squamous cells.
Additionally at the periphery of the mass, and occasionally surrounded
by the neoplastic squamous cells, were multifocal intramysial
nematode larvae identified as Trichinella sp. The larvae were
30-35 µm in diameter, coiled, and completely encapsulated
within the myofibers or nurse cells. Affected myofibers were focally
swollen to diameters of approximately 200 µm, which was
estimated to be 2 to 8 times the diameter of the adjacent normal
myofibers. The larvae were admixed with multiple myofiber nuclei
and were suspended within flocculent amphophilic intracytoplasmic
material in the nurse cells. The homogeneous capsules were 15-20
µm thick and were brightly eosinophilic, forming cysts around
the larvae. Occasional infected myofibers were surrounded by small
clusters of lymphocytes and plasma cells, with minimal numbers
of eosinophils in the infiltrates. Within the neoplastic tissue,
there were fewer encysted larvae than in myofibers at the tumor
periphery. These larvae, in foci of necrotic debris, were occasionally
degenerate, and small admixtures of neutrophils, macrophages and
necrotic debris surrounded the larvae. Occasional similar intact
larvae were within nurse cells within the skeletal muscle of the
diaphragm. The multifocal hepatic lesions were determined to be
benign cystic bile ductules.
Contributor's Diagnoses and Comments:
- 1. Lip, mucosa: Squamous cell carcinoma.
- 2. Lip, skeletal muscle: Myositis, subacute, moderate, multifocal,
with intramysial nematodes within nurse cells, etiology consistent
with Trichinella spiralis.
Trichinosis is a disease of humans and numerous other species
of warm- blooded animals caused by Trichinella spiralis and other
minor Trichinella species. The most common neoplastic condition
of the feline oral cavity is squamous cell carcinoma. Trichinosis
in association with neoplastic conditions has been reported only
rarely in the veterinary and human medical literature.
The life cycle of Trichinella sp. is unique among nematode
parasites. Larvae, present in skeletal muscle of carrier species,
excyst in the stomach and develop in the small intestine of the
host, undergoing four molts. Infected animals, therefore, act
as reservoirs of both intermediate and definitive stages of the
life cycle. Adult Trichinella species are 1 to 4 mm long, and
have intracellular and extracellular habitats within the small
intestine. The adults mate, and the ovoviviparous females deposit
the first stage larvae in the intestinal mucosa. The larvae penetrate
directly into the lymphatics, then enter the bloodstream to circulate
throughout the body, subsequently leaving the circulation to enter
the skeletal myofibers. For unexplained reasons, the larvae tend
to invade the myofibers of the masticatory and respiratory systems.
Larvae, while in the bloodstream, are vulnerable to removal by
the host immune system.
The incidence of human trichinosis has steadily decreased since
records were first kept in 1947, and the decreasing trend has
been attributed both to increased surveillance for the parasite
and improved hygiene. Larval Trichinella are identified by the
histological examination of infected host tissues, and by the
presence or absence of nurse cells. The first stage larvae of
T. spiralis induce the large nurse cells. Nurse cells are not
found in infections caused by the only other common species in
the genus, T. pseudospiralis. Trichinella spiralis and T. pseudospiralis
are the only two species that occur in the temperate areas of
the world. As the larvae in this case report were contained within
prominent nurse cells, it was felt that the morphology was most
consistent with T. spiralis. One source describes 8 genetically
different allotypes within the genus Trichinella, with 5 distinct
species and 3 taxonomic groups of uncertain status. In that report
the larvae were isolated from tissues obtained from a wide variety
of animals and humans on five continents. Adult Trichinella sp.
are identified morphologically, and the adults are usually obtained
from fecal specimens.
Associations between parasites and neoplastic disease have
been documented. The canine spirurid, Spirocerca lupi, has been
associated with esophageal fibrosarcoma and osteosarcoma. Cysticercus
fasciolaris, the larval stage of the cestode Taenia taeniaformis,
has been associated with fibrosarcoma in rats. In humans, neoplastic
disease has been linked to infestations by the trematodes Schistosoma
haemotobium (squamous cell carcinoma of the urinary bladder),
Clonorchis sinensis (cholangiocarcinoma), and Opisthorchis sp.
(cholangiocarcinoma). Of particular relevance with respect to
this case is the report of a 60-year-old man from Greece who was
diagnosed with concurrent trichinosis and oral and laryngeal squamous
cell carcinoma. A biopsy performed at the time of total laryngectomy
revealed numerous Trichinella larvae in the muscles of the larynx
and within neoplastic tissue, similar to the cat in this case
report. Also in the human case report, a small number of additional
cases of concurrent trichinosis and laryngeal squamous cell carcinoma
in humans were listed. In the human cases, as in our feline case,
the cause of the neoplastic condition remains conjectural, although
in the human cases, the chronic inflammation of the site by the
larval helminths was considered to be the probable cause of the
squamous cell carcinoma. It could also be possible that the increased
circulation to the site from neoplastic neovascularization may
have been the reason for the apparently preferential muscle tropism
and encystment of the T. spiralis larvae.
- Finally, the presence of the inflammatory and neoplastic
processes may have been entirely independent of one another,
especially considering that larvae were identified in the myofibers
of the diaphragm, where they were not associated with any neoplastic
change. That the two processes were independent of one another
was considered likely, as the preferential infestation of the
masticatory muscles by T. spiralis is documented, and squamous
cell carcinoma is the most common neoplastic process of the feline
oral cavity.
-
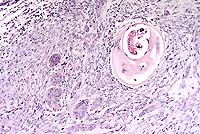
- Case 8-4. Lip (skin and mucosa). Encysted Trichinella
sp. in a myofiber and adjacents nests of invasive squamous cell
carcinoma with a dense fibroblastic (scirrhous) reaction. 10X
AFIP Diagnoses:
- 1. Haired skin: Squamous cell carcinoma, Domestic Shorthair
cat, feline.
- 2. Skeletal muscle, myocytes: Encysted nematode larvae, multiple,
consistent with Trichinella sp.
Conference Note: Within the neoplasm, some participants
noted the presence of pseudoglandular structures containing individualized
keratinocytes within their lumina. This finding is a feature of
the acantholytic squamous cell carcinoma, an uncommon variant
of squamous cell carcinoma described by Gross et al 7.
This case was reviewed by Chris Gardiner, PhD, veterinary parasitology
consultant to the AFIP. He believes it is virtually impossible
to speciate Trichinella sp. larvae in tissue section, but agrees
that the most common species is T. spiralis. Since these larvae
are aphasmids, their stichosome esophagus can be seen in section.
Dr. Gardiner noted that it is important to differentiate these
larvae from those of hookworms. Hookworm larvae will stay within
muscle cells for years, and will migrate to the mammary glands
when the queen begins lactating. However, hookworms are strongyles
and thus do not have stichosomes. Also in contrast to Trichinella,
hookworm larvae do not cause the muscle cell to form its "capsule".
Contributor: Veterinary Diagnostic Laboratory, Kansas State
University, 1800 Denison Avenue, Manhattan, Kansas 66506-5601
References:
- 1. Hulland TJ: Muscle and tendon. In: Pathology of Domestic
Animals, ed. Jubb KVF, Kennedy PC, Palmer N, 4th ed., Academic
Press, San Diego, California, vol.1, pp. 253-255, 1993.
- 2. La Rosa G, Pozio E, Rossi P, et al: Allozyme analysis
of Trichinella isolates from various host species and geographical
regions. J Parasitol 78:641-646, 1992.
- 3. Pozio E, La Rosa G, Murrell D, et al: Taxonomic revision
of the genus Trichinella. J Parasitol 78:654-659, 1992.
- 4. Simaskos N, Palaiologos Y, Eliopoulos PN: Trichinosis
and cancer of the larynx. J Laryngol Otol 106:171-172, 1992.
- 5. Stebbins KE, Morse CC, Goldschmidt MH: Feline oral neoplasia:
a ten-year study. Vet Pathol 26:121-128, 1989.
- 6. Wright KA: Trichinella spiralis: an intracellular parasite
in the intestinal phase. J Parasitol 65:441-445, 1979.
- 7. Gross TL, Ihrke PJ, Walder EJ: Veterinary Dermatopathology.
Mosby-Year Book, St. Louis, Missouri, p. 338, 1992.
International Veterinary Pathology Slide Bank:
Laser disc frame #2326, 9971, 12973, 13131, 18610, 18611, 19515
Terrell W. Blanchard
Major, VC, USA
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: blanchard@email.afip.osd.mil
* The American Veterinary Medical Association and the American
College of Veterinary Pathologists are co-sponsors of the Registry
of Veterinary Pathology. The C.L. Davis Foundation also provides
substantial support for the Registry.
Return to WSC Case Menu.



