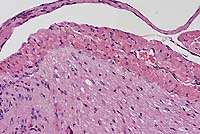
Case 6-1. Spinal cord. Ectopic skeletal muscle in meninges. 20X
AFIP Diagnosis: Spinal cord: Ectopic skeletal muscle, with focal necrosis, mouse, rodent.
Signalment: 125- to 180-day-old female or male hepatocyte growth factor/scatter factor transgenic mice on a FVB/N background.
History: Untreated. 5% of the mice developed hind limb paralysis.
Gross Pathology: None.
Laboratory Results: None.

Conference Note: Hepatocyte growth factor/scatter factor (HGF/SF) is a mesenchymally derived, multifunctional cytokine which in vitro stimulates proliferation, movement, and/or morphogenesis of a wide variety of epithelial cells expressing its tyrosine kinase receptor, Met. While HGF/SF is expressed in many tissues of mesenchymal origin, Met expression has been detected in the epithelium of almost all tissues. This suggests that under normal physiologic conditions HGF/SF functions as a paracrine regulator. Although the precise in vivo role of HGF/SF is still poorly understood, recent studies have shown that stimulation of the HGF/SF-Met signaling pathway is required for normal development of various tissues, including mammary gland and skeletal muscle.
Met has also been implicated in oncogenesis. The c-met gene is amplified in some carcinomas of the human gastrointestinal tract. It is also inappropriately expressed in such diverse human and murine tumors as melanoma, rhabdomyosarcoma, hepatoma, and mammary carcinoma. Paradoxically, however, HGF/SF has also been reported to inhibit the growth of certain carcinoma cell lines.2
As demonstrated by this case, transgenic mice which overexpress HGF/SF exhibit ectopic skeletal muscle in the central nervous system (CNS). Also in these mice, melanocytes were found in a number of ectopic sites, including the CNS. Moreover, HGF/SF overexpression stimulates hepatic proliferation, regeneration, and oncogenesis. HGF/SF has been implicated as a renotrophic agent, capable of stimulating renal regeneration after unilateral nephrectomy or acute kidney failure.4However, another recent study showed that mice overexpressing HGF/SF in the kidney and serum demonstrated prominent tubular cystic disease and progressive glomerulosclerosis, and were susceptible to premature death from renal failure.4
Contributor: National Cancer Institute, NCI-FCRDC, Fairview 201, P.O. Box B, Frederick, MD 21702-1201
References:
1. Takayama H, LaRochelle WJ, Anver M, Bockman DE, Merlino G. Scatter factor/hepatocyte growth factor as a regulator of skeletal muscle and neural crest development. Proc Natl Acad Sci USA 93:5866-5871, June 1996.
2. Takayama H, LaRochelle WJ, Sharp R, Otsuka T, Kriebel P, Anver M, Aaronson SA, Merlino G. Diverse tumorigenesis associated with aberrant development in mice overexpressing hepatocyte growth factor/scatter factor. Proc Natl Acad Sci USA 94:701-706, January 1997
3. Sakata H, Takayama H, Sharp R, Rubin JS, Merlino G, LaRochelle WJ. Hepatocyte growth factor/scatter factor overexpression induces growth, abnormal development, and tumor formation in transgenic mouse livers. Cell Growth & Differentiation 7:1513-1523, November 1996.
4. Takayama H, LaRochelle WJ, Sabnis SG, Otsuka T, Merlino G: Renal tubular hyperplasia, polycystic disease, and glomerulosclerosis in transgenic mice overexpressing hepatocyte growth/scatter factor. Lab Invest 77(2):131-138, August 1997.
International Veterinary Pathology Slide Bank:
Laser disc frame #: N/A
Signalment: 4-month-old, male, 129XC57BL mouse.
History: Injected intraperitoneally with acetaminophen
800mg/kg 48 hours previously.
Gross Pathology: None reported.
Laboratory Results: None.
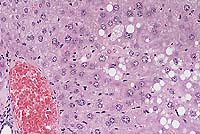
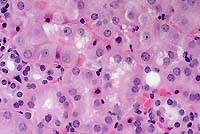
Conference Note: Conference participants discussed the distinguishing characteristics of the two morphologic expressions of cell death, necrosis and apoptosis.
Necrotic cells show early cytoplasmic swelling and increased eosinophilia, progressing to a more glassy homogeneous appearance than that of normal cells. When cytoplasmic organelles have been enzymatically digested, the cytoplasm becomes vacuolated and appears moth-eaten. Calcification of necrotic cells may occur. Nuclear changes appear in three patterns: karyolysis, a fading of the basophilia of the chromatin reflecting DNAse activity; pyknosis, characterized by nuclear shrinkage and increased basophilia; karyorrhexis, the fragmentation of a pyknotic or partially pyknotic nucleus.
Apoptosis is responsible for cell death in several important physiologic, and some pathologic, processes. These include: 1) programmed destruction of cells during embryogenesis; 2) hormone-dependent physiologic involution; 3) cell depletion in proliferating populations, including tumors; 4) death of lymphocytes after cytokine depletion; and others. The following morphologic features characterize cells undergoing apoptosis:
1) Cell shrinkage.
2) Chromatin condensation. The chromatin aggregates peripherally,
under the nuclear membrane, into well-delineated dense masses
consisting of 180-200 base pairs. This is the most chaacteristic
feature of apoptosis.
3) Formation of cytoplasmic blebs and apoptotic bodies. Apoptotic
bodies are membrane-bound structures containing cytoplasm and
tightly packed cellular organelles, with or without a nuclear
fragment.
Apoptotic bodies are rapidly phagocytized and degraded by adjacent healthy cells. In contrast to necrosis, apoptosis does not elicit inflammation. Also, apoptosis usually involves single cells or small clusters of cells. These factors lead to difficulty in detecting apoptosis histologically.
There is considerable interest in the toxicity of acetaminophen, both because of its extensive clinical use and its usefulness in studying mechanisms of hepatotoxicity. This toxicity is believed to be mediated by a highly reactive arylating metabolite formed by the cytochrome P-450-dependent mixed function oxidase system, which is also present in high concentrations in renal proximal convoluted tubular epithelium. After detoxification pathways are impaired and the metabolite is formed in excessive amounts, it can react and covalently bind to cellular macromolecules, causing cellular injury and ultimately leading to cell death. Ray, et al4, recently showed that both apoptosis and necrosis contribute significantly to acetaminophen hepatotixicity in vivo.
Contributor: National Cancer Institute, NCI-FCRDC, Fairview 201, P.O. Box B, Frederick, MD 21702-1201
References:
1. Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie
BB: Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism.
Journal of Pharmacology and Experimental Therapeutics 187(1):185-194,
1973.
2. Hoivik DJ, Manautou JE, Tveit A, Hart SGE, Khairallah EA, Cohen SD: Gender- related differences in susceptibility to acetaminophen-induced protein arylation and nephrotoxicity in the CD-1 mouse. Toxicology and Applied Pharmacology 130:257- 271, 1995.
3. Hart SGE, Cartun RW, Wyand DS, Khairallah EA, Cohen SD: Immunohistochemical localization of acetaminophen in target tissues of the CD-1 mouse: Correspondence of covalent binding with toxicity. Fundam. Appl. Toxicol. 24:260-274, 1995.
4. Ray SD, Mumaw VR, Raje RR, Fariss MW: Protection of acetaminophen- induced hepatocellular apoptosis and necrosis by cholesteryl hemisuccinate pretreatment. Journal of Pharmacology and Experimental Therapeutics 279:1470- 1483, 1996.
5. Cotran RS, Kumar V, Robbins SL: Robbins Pathologic Basis of Disease, 5th ed., W.B. Saunders Company, 1994, pp. 13-20.
6. Kumar V, Cotran RS, Robbins SL: Basic Pathology, 6th ed., W.B. Saunders Company, 1997, pp. 13-14.
International Veterinary Pathology Slide Bank:
Laser disc frame #: N/A.
Signalment: A 2-month-old normal female laboratory mouse is submitted. The background is B10.A(2R)/SgSnJ.
History: The mouse is normal and submitted to illustrate a strain specific feature.
Gross Pathology: The cranial portion of the spleen has a discreet black focus present.
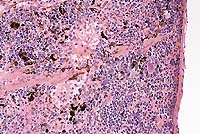
Conference Note: Hemosiderin and melanin can look similar in histologic sections of spleen, and can be present simultaneously. Melanin-containing cells in the spleen sometimes have a "stringy" appearance due to the presence of one or more dendritic processes. The gross observation that all or part of a tissue is black is a helpful diagnostic aid.
In this case, the Perls iron stain was negative and the Fontana-Masson method for melanin was positive.
Contributor: The Jackson Laboratory, 600 Main Street, Bar Harbor, ME 04609-1500.
References:
1. Van der Heijden A, Van Dijk JE, Lemmens AG, Beynen AC: Spleen
pigmentation in young C57BL mice is caused by accumulation of
melanin. Laboratory Animals 29:459-463, 1995.
2. Veninga TS, Wiering RA, Morse H: Pigmented spleens in C57BL mice. Laboratory Animals 23:16-20, 1989.
3. Weissman I: Genetic and histochemical studies on mouse spleen black spots. Nature 215(5098):315, 1967.
4. Sundberg JP: Letters to the editor: pigmented spleens in C57BL mice. Laboratory Animals 25:85-86, 1991.
5. Lyon MF, Searle AG: Bsp, black spleen, dominant. Genetic variants and strains of the laboratory mouse. Second edition, Oxford University Press, Oxford, p. 58, 1990.
International Veterinary Pathology Slide Bank:
Laser disc frame #8664-7.
Signalment: 7-week-old rabbit.
History: The owner noted swollen livers when butchering rabbits. One rabbit was ataxic and all rabbits had poor weight gain with swollen abdomens.
Gross Pathology: Not provided.
Laboratory Results: N/A.
Contributor's Diagnoses and Comments:
The lesions are characteristic of hepatic coccidiosis in rabbits caused by Eimeria stiedae. The granulomatous hepatitis observed in some of the sections was presumably the result of a foreign-body reaction. The cause of the acute hepatic necrosis observed in some of the sections was not determined.
Hepatic coccidiosis primarily affects weanling rabbits. The primary manifestations range from subclinical disease to poor weight gain to death. At necropsy, the liver is enlarged and has multiple pale yellow foci.
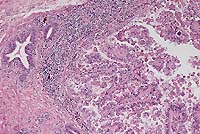
Conference Note: Significant variations in liver enzymes and serum chemistry values have been observed in the course of experimental infection with E. stiedae. Four pathophysiologic stages were delineated by Barriga and Arnoni1:
1) An initial stage, coinciding with anti-E. stiedae antibody
production, characterized by a sustained rise in serum transaminases.
2) A cholestatic period, with elevated serum conjugated (direct)
bilirubin, coinciding with the beginning of oocyst production.
3) A stage of metabolic alterations, characterized by hypoproteinemia
and hypoglycemia.
4) A phenomenon of immunosuppression that inhibits the ability
of the host to restrict the production of the sexual stages of
the parasite.
In addition to hepatic disease, rabbits also are susceptible to
enteric coccidiosis. At least 12 species of Eimeria have been
described in the intestinal tract of domestic and wild rabbits.
Contributor: Texas Veterinary Medical Diagnostic Laboratory, Post Office Drawer 3040, College Station, TX 77841
International Veterinary Pathology Slide Bank:
Laser disc frame #5286, 7260, 13123, 19739, 20405-10, 21515-17,
Terrell W. Blanchard
Major, VC, USA
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: blanchard@email.afip.osd.mil
* The American Veterinary Medical Association and the American College of Veterinary Pathologists are co-sponsors of the Registry of Veterinary Pathology. The C.L. Davis Foundation also provides substantial support for the Registry.