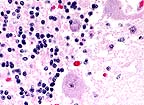
 Multiple eosinophilic intracytoplasmic
inclusion bodies (Negri bodies) in the cells of the Purkinje layer
in the cerebellum of a 6-month old foal. (HE, 400X, 59K)
Multiple eosinophilic intracytoplasmic
inclusion bodies (Negri bodies) in the cells of the Purkinje layer
in the cerebellum of a 6-month old foal. (HE, 400X, 59K)Signalment: 6-month-old foal.
History: Exhibited aggressive behavior with intermittent convulsions for 2 days prior to death.
Gross Pathology: None.
Laboratory Results: Sections of cerebellum are positive by florescent antibody testing for rabies virus.
Contributor's Diagnosis and Comments: Encephalitis, lymphocytic, with neuronal intracytoplasmic eosinophilic inclusions, cerebellum.
Etiology: Rabies virus
In the cerebrum, occasional vessels are surrounded by thin perivascular cuffs of lymphocytes. The cerebral cortex is mildly hypercellular (glial cells) and occasional neurons contain eosinophilic intracytoplasmic inclusion bodies that vary from 2-7 æm in diameter. In sections of cerebellum there is segmental loss of Purkinje cells; many remaining Purkinje cells contain single to multiple eosinophilic inclusions similar to those seen in cortical neurons.
The inflammatory response in the brain is typical of a viral encephalitis. The presence of intracytoplasmic bodies within neurons is most compatible with rabies virus infection. While differential etiologic diagnoses of non-suppurative encephalitis in horses must include arboviral encephalitis, multiple intracytoplasmic inclusion of the type observed are not described with western, eastern or Venezuelan encephalitis. Brain samples forwarded to the Center for Disease Control are positive by fluorescent antibody testing for rabies virus antigen. Source of the virus and route of exposure are unknown.
AFIP Diagnosis: Cerebellum: Encephalitis, nonsuppurative, minimal, with neuronal eosinophilic intracytoplasmic inclusion bodies, breed not specified, equine, etiology consistent with rabies virus.
Conference Note: Rabies virus belongs to the genus Lyssavirus in the family Rhabdoviridae, and causes a lethal nonsuppurative encephalomyelitis, ganglionitis, and parotid adenitis in warm-blooded vertebrates. It is an extremely important zoonotic disease. In some nations such as India and the Philippines, rabies accounts for thousands of human deaths each year.
Horses are one of the most susceptible domestic animal species and are usually infected via a bite wound inflicted by a rabid animal. Replication occurs first in myocytes near the site of inoculation. Viral particles bud from the plasma membrane and enter local neuromuscular and neurotendinous spindles and ascend to the central nervous system via axoplasmic flow. Rabies virus has an affinity for binding nicotinic acetylcholinergic receptors; this may be the mechanism for passage of virus between nerves and neurons. Upon reaching the central nervous system, replication continues and the virus spreads centrifugally along sensory fibers or specific motor fibers to the adrenal gland, lacrimal and salivary glands, kidney, pancreas, and nasal mucosa. Virus buds from the plasma membrane of mucous acinar cells of the salivary glands and is shed directly into salivary secretions a few days before the onset of clinical signs. There is eventual widespread dissemination within the brain causing depression and coma with death usually resulting from respiratory arrest.
Histologically, rabies infection is characterized by a nonsuppurative encephalomyelitis and ganglioneuritis with intraneuronal eosinophilic inclusion bodies (Negri bodies). The typical reaction is perivascular cuffing, gliosis and neuronal degeneration. The microscopic findings can vary greatly from case to case and from species to species with carnivores generally showing a more severe reaction than herbivores. In this case, inflammation is minimal. Negri bodies are generally 2-8 mm, discrete to amorphous inclusions with a clear, thin halo that range from none to several per cell. The location of neurons that contain Negri bodies is important and differs with the species of animal affected. Generally, the hippocampus is the most common site for finding Negri bodies in carnivores, whereas the Purkinje cells of the cerebellum are most often affected in herbivores and humans. Negri bodies have also been found in ganglion cells of the adrenal medulla, retina, and salivary gland. In some species, care must be taken to differentiate Negri bodies from nonspecific inclusions that have been found in the pyramidal cells of the hippocampus or the lateral geniculate nucleus neurons in cats, skunks, and dogs and in the larger neurons of the medulla and spinal cord of old sheep and cattle; these inclusions are often smaller than Negri bodies. Dogs also may have cytoplasmic lamellar bodies in neurons of the thalamus and cerebellar cortical Purkinje cells.
The classical histologic lesions of rabies have been expanded to include a rabies-induced spongiform change seen in the gray matter of the brains of experimentally-infected skunks and foxes and more recently, in a naturally infected heifer. This spongiform change was likened to the lesions of other spongiform encephalopathies such as scrapie, bovine spongiform encephalopathy, etc.
Contributor: Department of Veterinary Microbiology and Pathology, Washington State University, Pullman, WA 99164-7040.
References:
1. West, GP: Equine rabies. Equine Vet J, 17:280-282, 1985.
2. Hamir, AN; Moser G; Rupprecht CE: A five year (1985-1989) retrospective study of equine neurological diseases with special reference to rabies. J of Comp Pathol, 106:411-421, 1992..
3. Green SL: Equine rabies, Vet Clin of N Am, Equine Practice, 9:337-347, 1993.
4. Jubb KVF; Kennedy PC; Palmer N. (eds): Pathology of Domestic Animals, 4th ed., pp.403-406, 1993.
5. Foley GL; Zachary JF: Rabies-induced Spongiform Change and Encephalitis in a Heifer, Vet Pathol 32:309-311, 1995.
6. Summers BA; Cummings JF; de Lahunta A (eds): Veterinary Neuropathology, Mosby-Year Book, pp. 95-99, 1995.
International Veterinary Pathology Slide Bank: Laser disc frame #582, 4741, 5243, 5282, 9705, 14209-11, 16939, 17065, 21489, 21491-2, 22265, 24118-22.
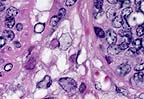 Higher magnification of the
above field. (HE, 400X, 50K).
Higher magnification of the
above field. (HE, 400X, 50K).
Signalment: Tissue from a one year old Hereford steer experimentally inoculated intradermally with the Neethling strain of lumpy skin disease virus.
History: One week postinoculation, the animal became febrile and developed dermal nodules multifocally. In addition, there were some target-like nodular areas of the muzzle. There was some dyspnea.
Gross Pathology: The most significant lesions were restricted to the skin. There was an extensive distribution of dermal nodules, some of which had necrotic centers and were very excoriating in appearance. Multiple areas of consolidation were scattered throughout the pulmonary parenchyma, but most prominently in subpleural areas.
Laboratory Results: None.
Contributor's Diagnosis and Comments: Dermatitis, subacute to chronic, severe, with vasculitis and "sheep pox cells", pinna.
Etiology: Capripoxvirus, specifically lumpy skin disease virus.
Lumpy skin disease virus is classified with two closely related viruses, sheep pox and goat pox, in the Capripoxvirus genus of the family Poxviridae. All three viruses cause similar clinico-pathologic syndromes in the respective species, with widespread cutaneous eruptions often accompanied by dissemination to internal organs, especially lung.
Transmitted principally by biting flies and contact, lumpy skin disease is endemic in much of Africa. It is generally thought to be a disease of high morbidity and low mortality.
Grossly, the skin lesions appear as variably sized, marked, firm swellings. With time, the centers of some of the swellings will become necrotic and ulcerated, resulting in a characteristic lesion known as a "sitfast." Histologically, the skin lesions are very distinct and share features in common with the other capripoxviruses, i.e. sheep pox and goat pox. There is often a striking paucity of epidermal changes. The dermis is edematous, cellular, and occasionally infarcted. Scattered throughout the inflammation are variable numbers of "sheep pox cells" - histiocyte-like cells with large vacuolated nuclei and poorly-defined eosinophilic cytoplasmic inclusions. In addition to skin, these "sheep pox cells" may be seen in other tissues altered by the virus such as nasal turbinates, trachea, and lung.
AFIP Diagnosis: Haired skin, pinna: Dermatitis, periadnexal, necrotizing, subacute, multifocal, moderate, with necrotizing vasculitis and intrahistiocytic eosinophilic cytoplasmic inclusions, Hereford, bovine, etiology consistent with lumpy skin disease virus.
Conference Note: Lumpy skin disease (LSD) is of major economic importance because of the prolonged debilitating effect it may have on animals with losses resulting from emaciation, temporary or permanent cessation of milk production, infertility in bulls and cows, mastitis, abortion, and permanent damage to hides.
Subcutaneous or intradermal inoculation of LSD virus results in the development of localized swelling at the site of inoculation after four to seven days and enlargement of the regional lymph nodes. Generally, eruption of skin nodules occurs 7 to 19 days post-inoculation. Viremia occurs after an initial febrile period and persists for about four days. A variety of cell types, including epithelial and endothelial cells, pericytes and fibroblasts are infected by the virus. Vasculitis can result from viral infection of endothelium, pericytes and probably other cells in blood and lymph vessels. Infarction may occur in severe infections. LSD virus is present in skin nodules, normal appearing skin, lymph nodes, liver, kidneys, skeletal muscle, saliva, and semen of infected animals.
Nodules within the skin and subcutis are the most characteristic finding; however, most affected animals have multifocal, roughly circular, necrotic areas on the muzzle and in the respiratory tract, buccal mucosa, forestomachs, abomasum, uterus, vagina, teats, udder, and testes. Generalized lymphadenopathy is also a common finding. Skin lesions must be differentiated from lesions caused by pseudo-lumpy skin disease, insect bites, ringworm, actinomycosis, nocardiosis, streptomycosis, dermatophilosis, demodicosis, onchocerciasis, and besnoitiosis. Differential diagnosis for the mucosal lesions includes bovine viral diarrhea, rinderpest, malignant catarrhal fever, bovine papular stomatitis, and bluetongue.
Contributor: Foreign Animal Disease Diagnostic Lab, USDA-APHIS, P.O. Box 848, Greenport, NY 11944.
References:
1. House JA; Wilson TM; El Nakashly S; Karim IA; Ismail I; El Danaf N; Moussa Am; Ayoub NN: The isolation of lumpy skin disease virus and bovine herpesvirus-4 from cattle in Egypt. J of Vet Diag Invest 2:111-115, 1990.
2. Woods JA: Lumpy skin disease - A review. Tropical Animal Health and Production 20:11-17, 1988.
3. Coetzer JAW; Thomson GR; Tustin RC, (eds): Infectious disease of livestock, with special reference to Southern Africa. Oxford, pp. 604-612, 1994.
International Veterinary Pathology Slide Bank: Laser disc frame #12465-72, 21949-60.
Signalment: Placenta of stillborn female gazelle born to a 3-year-old female Cuvier's gazelle (Gazella cuvieri)
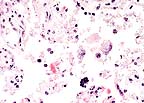 Placental necrosis in a 3-year-old
Cuvier's gazelle (HE, 100X, 31K).
Placental necrosis in a 3-year-old
Cuvier's gazelle (HE, 100X, 31K).
History: Late term abortions and stillbirths in 3 females in this herd within a 3 month period.
Gross Pathology: No gross lesions.
Laboratory Results: None performed.
Contributor's Diagnosis and Comments: Severe acute necrotizing placentitis with intracellular organisms (Coxiella burnetii)-placenta-Cuvier's gazelle.
Significant lesions were limited to the placenta. Several epithelial cells in areas of necrosis have fuzzy blue cytoplasm. Sections were stained with Gimenez method, which revealed <1 micron diameter red organisms within the cytoplasm. Organisms did not stain with Brown-Brenn Gram stain. Paraffin blocks were submitted to Washington Animal Disease Diagnostic Laboratory for immunohistochemistry. Sections were positive for Coxiella and negative for Chlamydia.
Coxiella burnetii is a ubiquitous organism. It causes respiratory disease (Q fever) in humans and abortion (typically late in gestation) in domestic cattle, sheep and goats. Exposure to, or infection with, this agent has been documented in a wide range of species of mammals, birds, reptiles and amphibians, but infections in gazelles has not been reported. Infection occurs from exposure to contaminated fluids (placenta, milk) or dust. These gazelles were housed by themselves in a separate exhibit, and the source of infection case has not yet been determined.
AFIP Diagnosis: Placenta: Placentitis, necrotizing, acute, multifocal, moderate, with focal vasculitis and intracellular organisms, Cuvier's gazelle (Gazella cuvieri), artiodactyla.
Conference Note: Microscopically, placental trophoblasts lining the cotyledonary villi are distended by small, approximately 1æm diameter, basophilic, intracytoplasmic organisms. The differential diagnosis for intratrophoblastic organisms in cases of placentitis includes Coxiella, Brucella, Campylobacter, and Chlamydia. Of these, only Coxiella burnetii and Chlamydia stain positively with Gimenez or modified acid-fast stains. The morphology of the intracytoplasmic organisms on Gimenez-stained sections should differentiate C. burnetii from Chlamydia since C. burnetii appear as pleomorphic, or thin, rod-shaped structures, while chlamydial elementary bodies are uniformly small and round. Some, but not all, sections examined by conference participants had a focal vasculitis of one of the larger placental vessels.
Contributor: Zoological Society of San Diego, Department of Pathology, P.O. Box 551, San Diego, CA 92112-0551.
References:
1. Bell JF: Q Fever, In: Infectious Diseases of Wild Mammals,
ed. Davis JW, Karstad LH, Trainer DO, 2nd ed., pp. 388-397. Iowa
State Univ. Press, Ames, Iowa, 1981.
2. Dilbeck PM; McElwain TF: Immunohistochemical detection of Coxiella burnetii in formalin-fixed placenta. J Vet Diagn. Invest 6:125-127, 1994.
3. Moore JD; Barr BC; Daft BM; O'Connor MT: Pathology and Diagnosis of Coxiella burnetii Infection in a Goat Herd, Vet Pathol 28:81-84, 1991.
International Veterinary Pathology Slide Bank: None.
Signalment: Adult gelding presented for slaughter.
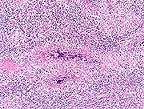 Group of H. deletrix
larva within the granulomatous inflammation in a horse kidney.
(HE, 200X, 83K)
Group of H. deletrix
larva within the granulomatous inflammation in a horse kidney.
(HE, 200X, 83K)
History: No previous history available. No antemortem findings.
Gross Pathology: Submitting inspection veterinarian noted bilateral, discrete, tannish-white, homogeneous nodules approximately 1 inch in diameter that bulged on cut surface and were the same texture as that of surrounding renal tissue.
Laboratory Results: None.
Contributor's Diagnosis and Comments: Chronic, severe multifocal granulomatous nephritis with intralesional rhabditiform nematodes, consistent with Halicephalobus deletrix.
Morphologic Description: There are multifocal to coalescing foci replacing the renal parenchyma. The foci contain cross and longitudinal sections of a nematode with a smooth cuticle, pseudocoelom, a tubular digestive tract lined by low cuboidal epithelium, tapered tail, and rhabditiform esophagus with a corpus:isthmus:bulb ratio of 3:2:1. Mature females, larvae, and eggs are present, and females rarely contain ova in various stages of development. The parasite is surrounded by a mixture of fibrin, neutrophils, cellular debris, epithelioid macrophages and multinucleated giant cells that are contiguous with coalescing infiltrates of plasma cells, lymphocytes, and eosinophils in a loose fibrovascular stroma. Occasional tubules are present among the inflammatory cells, and these are often lined by necrotic or regenerating epithelial cells and contain proteinic and cellular debris.
H. deletrix is distinguished by a uterus that is dorsoflexed at the ovary and ventroflexed at the vulva, a tapered tail, and a rhabditid esophagus with a corpus:isthmus:bulb ratio of 3:2:1. All eight species are considered free-living nematodes found in soil and decaying organic material. However, the free-living stages of H. deletrix have not been identified, and only adult females, larvae, and eggs have been reported from tissues of horses and human beings. The brain is the most commonly involved tissue in horses, followed by the kidneys, oral and nasal cavities, lymph nodes, lungs, liver, ganglia, bone and eye. The parasite may induce an acute syndrome of cerebral vasculitis and hemorrhagic necrosis, as well as granulomatous lesions in brain and other tissues. Renal infections produce cream-colored masses which may resemble neoplasms grossly. The method of infection is unknown. It is presumed that infection occurs by contamination of mucosal, cutaneous, or ocular wounds by larvae in the environment, with hematogenous dissemination accounting for the high incidence of kidney and brain involvement. In addition, pulmonary infection in two foals has lead to the suspicion that infection may occur by inhalation, transplacental, or transmammary routes.
AFIP Diagnosis: 1. Kidney: Nephritis, granulomatous, focally extensive, severe, with numerous adult and larval rhabditid nematodes, breed not specified, equine, etiology consistent with Halicephalobus deletrix. 2. Kidney: Nephritis, interstitial, chronic, multifocal, mild to moderate, with interstitial fibrosis and tubular dilatation.
Conference Note: Conference participants agreed with the contributor's diagnosis and comments and that the etiology is consistent with Halicephalobus deletrix. The differential diagnosis includes Halicephalobus, Strongyloides and Cephalobus. All three have a rhabditiform esophagus; however, only Halicephalobus has a reflexed ovary and a pointed tail.
Contributor: United States Department of Agriculture, Food Safety and Inspection Service, Science-Pathology, Eastern Laboratory, Russell Research Center, P.O. Box 6085, College Station Road, Athens, GA, 30604.
References:
1. Spalding MG; Greiner EC, Green SL: Halicephalobus (Micronema) deletrix infection in two half-siblings foals. J Am Vet Med Assoc 196:1127-1129, 1990.
2. Rames DS; Miller DK; Barthel R; Craig TM; Dziezyc J; Helman RG; Mealey R: Ocular Halicephalobus (syn. Micronema) deletrix in a Horse. Vet Pathol 32:540-542, 1995.
3. Blunden AS; Khalil LF; Webbon PM: Halicephalobus deletrix infection in a horse. Equine Vet J 19:255-260, 1987.
4. Dunn DG; Gardiner CH; Dralle KR; Thilsted JP: Nodular Granulomatous Posthitis Caused by Halicephalobus (syn. Micronema) sp. In a Horse. Vet Pathol 30:207-208, 1993.
International Veterinary Pathology Slide Bank: Laser disc frame #3654, 3655, 3656, 6270, 6346, 6612, 6613, 6614, 7942.
* The American Veterinary Medical Association and the American College of Veterinary Pathologists are co-sponsors of the Registry of Veterinary Pathology. The C.L. Davis Foundation also provides substantial support for the Registry.