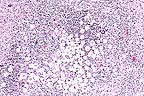
 Granulomatous encephalitis
with the classic "soap-bubble" appearance associated
with Cryptococcus neoformans in the cerebrum of a horse.
(HE, 100X, 65K)
Granulomatous encephalitis
with the classic "soap-bubble" appearance associated
with Cryptococcus neoformans in the cerebrum of a horse.
(HE, 100X, 65K)Signalment: 3-year-old Belgian gelding.
 Granulomatous encephalitis
with the classic "soap-bubble" appearance associated
with Cryptococcus neoformans in the cerebrum of a horse.
(HE, 100X, 65K)
Granulomatous encephalitis
with the classic "soap-bubble" appearance associated
with Cryptococcus neoformans in the cerebrum of a horse.
(HE, 100X, 65K)
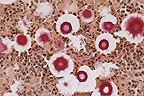 Carmininophilic yeasts in
the cerebrum of a horse. The clear "halo" around each
yeast is due to shrinkage of the capsule during fixation. (Mucicarmine,
400X, 40K)
Carmininophilic yeasts in
the cerebrum of a horse. The clear "halo" around each
yeast is due to shrinkage of the capsule during fixation. (Mucicarmine,
400X, 40K)
History: On May 29, 1995, the owner noted that the gelding had a head tilt. Three days later it was presented to the veterinary teaching hospital with a head tilt and was circling to the right. The following day, the horse was down in the stall, unable to rise, and later began to have convulsions. It was euthanized and presented for necropsy.
Gross Pathology: One half of the brain was submitted to the state laboratory to be tested for rabies. There were several firm tan/gray nodules randomly scattered throughout the lungs. The largest was irregular in shape, measured 5 x 8 x 3 cm, and was located in the right caudal lobe. The stomach contained numerous Gastrophilus sp. larvae. The small intestines contained abundant Parascaris sp. Several Anoplocephala sp. were attached to the ileo-cecal valve. There were several yellow-tan malacic foci randomly scattered throughout the midbrain. Those foci ranged from 1-12 mm in diameter.
Laboratory Results: FA test for rabies was negative.
Contributor's Diagnosis and Comments: Encephalitis, pyogranulomatous, multifocal, severe: Cerebral cryptococcosis, due to infection with Cryptococcus neoformans.
The brain contained numerous encapsulated yeast bodies compatible with C. neoformans. Similar organisms were present in the lung within the grossly visible nodules. Lesions in the lungs were those of pyogranulomatous pneumonia. This case is somewhat unusual, because in the CNS 1) C. neoformans is usually associated with meningitis and 2) the organism usually evokes minimal inflammatory response. In this case, lesions were confined to the midbrain; the meninges were spared.
AFIP Diagnosis: Midbrain (per contributor): Encephalitis, granulomatous, multifocal to coalescing, moderate, with yeast, Belgian, equine, etiology consistent with Cryptococcus neoformans.
Conference Note: Cryptococcus neoformans is a yeast-like fungus in tissue. It reproduces by single buds, is 4-8 æm in diameter, and is surrounded by a thick mucopolysaccharide capsule. The capsule stains well with mucicarmine and alcian blue and is PAS-positive. Cryptococcosis occurs worldwide; the source of the infection is believed to be soil, especially in areas contaminated by pigeon or other bird feces. All species of animals can be affected, but cats are affected more often than other domestic animals.
The respiratory tract is the usual site of primary infection, the nasal cavity being affected more often than the lungs. From the respiratory tract, the yeast can disseminate by hematogenous routes throughout the body; however, it has a predilection for the central nervous system. In addition, from nasal lesions, there is potential for local spread to the meninges and brain.
Histologically, the lesion is described as having a "soap-bubble" appearance, the result of the thick, nonstaining, capsule. The inflammatory response to cryptococcosis is usually minimal, consisting of macrophages, lymphocytes, and plasma cells. Occasionally, a granulomatous reaction will develop, especially in the lungs.
Cutaneous cryptococcosis is also common in the cat and occurs as either a primary site of infection or as the result of metastasis from systemic lesions. Inflammatory cutaneous lesions appear as small, firm nodules, which tend to ulcerate and drain large amounts of serous fluid. In cats, the head is most frequently affected, although lesions may be distributed widely over the body.
Contributor: Department of Veterinary Pathology, College of Veterinary Medicine, Oklahoma State University, Stillwater, OK 74078.
References:
1. Welsh, R.D. and Stair, E.L. Cryptococcal meningitis in a horse.
J Eq Vet Sci 15:80-82, 1995.
2. Steckel, R.R., Adams, S.B., Long, G.G., and Rebar, A.H. Antemortem diagnosis and treatment of crytococcal meningitis in a horse. J Am Vet med Assn 180:1085-1089, 1982.
3. Dungworth DL: The respiratory system in Pathology of domestic animals. Jubb KVF, Kennedy PC, and Palmer N eds., Academic Press, Inc., San Diego, 4th edition, vol. 2, pp. 667-668, 1993.
International Veterinary Pathology Slide Bank: Laser disc frame #771, 1329, 1648-50, 1949-52, 2580, 4240, 5916, 6293, 6440, 6968, 8126-27, 8332-34, 10078, 11902, 14452, 19343-46, and 20953.
Signalment: 3-year-old female llama.
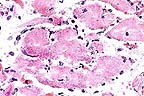 Myocardial necrosis and mineralization
of myofibers in a llama which had ingested oleader. (HE, 400X,
63K)
Myocardial necrosis and mineralization
of myofibers in a llama which had ingested oleader. (HE, 400X,
63K)
History: The llama was found dead. It was housed in a pen next to the toxic plant area on campus and had access to the toxic plants.
Gross Pathology: The first stomach compartment contained large amounts of hay and fragments of leaves. Some leaves were consistent with oleander. There were marked multifocal ecchymoses over the entire epicardial surface.
Laboratory Results: Toxicology - The stomach and cecal contents contained 0.05 ppm oleandrin, the toxic principle of oleander.
Contributor's Diagnosis and Comments: Myocardial degeneration and necrosis, marked, multifocal, acute (Etiology: oleander (Nerium oleander) toxicosis).
Oleander (Nerium oleander) is a common ornamental hedge in the Western and Southern United States. The toxic compound is a cardiac glycoside, similar to digitoxin. Cardiac glycosides act by inhibiting the sodium/potassium-ATPase pump which is active in maintaining the transmembrane potential of cardiac muscle fibers. The toxic principle is found in all parts of the plant but is most concentrated in the seeds. The lethal dose is low with less than 35 grams of dried leaves being a toxic dose for a 150 kg llama. Oleander toxicity also occurs in horses and cattle.
Oleander toxicity causes profuse watery or bloody diarrhea due to increased gastrointestinal motility. The glycoside has a direct effect on cardiac muscle and the effects include an increased force of contraction, decreased rate of contraction, increased myocardial excitability and increased automaticity. Clinically, tachycardia progresses to ventricular fibrillation and death. Clinical signs can begin to develop 5-24 hours after ingestion and death can occur from 10 hours to 3-4 days.
At necropsy, affected animals often have a severe catarrhal or hemorrhagic gastroenteritis and terminal hemorrhages affecting the myocardium and other serosal and mucosal surfaces. If the ingested dose is high, an animal may die of cardiac complications before diarrhea develops. Additional necropsy findings in horses can include pericardial effusion, subcutaneous edema, and focal myocardial pallor. The histologic lesion is multifocal myocardial degeneration and necrosis.
AFIP Diagnosis: Heart: Necrosis, multifocal, extensive, with subacute myocarditis, mineralization, and subepicardial hemorrhage, llama (Lama glama), camelid.
Conference Note: Poisoning with cardiac glycosides is acute, rather than cumulative, and death usually occurs shortly after ingestion of the toxic plant. Plants containing cardiac glycosides are usually unpalatable and are often ingested when clippings from ornamental plants are accidentally provided to animals. Other plants that contain cardiac glycosides and have been responsible for significant losses in livestock include Homeria spp. (the cape tulip), Thevetia peruviana (yellow oleander), Tylecodon spp., and Bryophyllum tubiflorum. Humans have been fatally poisoned by ingesting digitalis-containing plants such as lily-of-the-valley (Convallaria majalis), foxglove (Digitalis purpurea), and oleander.
Increased contractility of cardiac muscle occurs when cardiac glycosides bind and inhibit the sodium-potassium-ATPase pump, which is embedded in the plasma membrane of the cardiomyocyte. Binding prevents extrusion of Na+ and intake of K+. This causes an increase in the intracellular level of Na+, and because muscle cells can exchange Ca+ for Na+ there is a net increase in intracellular Ca+. The increase in calcium facilitates contraction of myofilaments causing the clinical signs and histologic lesions associated with cardiac glycoside intoxication.
Contributor: University of California, Dept of Pathology, Veterinary Medical Teaching Hospital, Davis, CA, 95616.
References:
1. Fowler, M.E. Medicine and Surgery of South American Camelids.
1989. Iowa State University Press.
2. Jubb, K.V.F., P.C. Kennedy and N. Palmer. Pathology of Domestic Animals. Vol. 3, pg 28. 1993. Academic Press, Inc.
3. Siemens, L.M., F.D. Galey, B. Johnson and W.P. Thomas. "The Clinical, Cardiac and Pathophysiological effects of Oleander Toxicity in Horses." From UC Davis VMTH House Officer Seminar Day Proceedings, March 1995.
4. Smith, B.P. Large Animal Internal Medicine. 1990. The C.V. Mosby Company.
International Veterinary Pathology Slide Bank: Laser disc frame #14114.
Signalment: 8-week-old male turkeys. There were 12,000 birds in this flock.
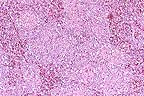 Marked reticuloendothelial
hyperplasia in a turkey with adenoviral splenitis. Note the absence
of detectable lymphoid elements. (HE, 100X, 86K)
Marked reticuloendothelial
hyperplasia in a turkey with adenoviral splenitis. Note the absence
of detectable lymphoid elements. (HE, 100X, 86K)
History: - Mortality had been increasing for last 3 days - Coban and Stafac provided in feed - Moved to grower barn at 6 weeks of age; environment is good
Gross Pathology: Birds were dehydrated and had congestion of breast musculature. Some had feed in crops. Livers were firm, congested, and dotted with petechial hemorrhages. Spleens were very enlarged and congested.
Laboratory Results: Large numbers of Escherichia coli were recovered from blood filtering organs. Transmission electron microscopy (TEM) of spleen confirmed the presence of intranuclear adenoviral particles, 80 nm in diameter, within cells. These virions are widely scattered throughout the enlarged nuclei. There is margination of chromatin.
Contributor's Diagnosis and Comments:
Morphologic Diagnosis: Moderate acute lymphocytolysis, reticular cell and macrophage hyperplasia with intranuclear viral inclusions, mild multifocal acute splenic fibrinous necrosis, splenic heterophilia.
Etiology: Adenovirus type II. Name of the disease: Hemorrhagic enteritis.
Colibacillosis is an important and costly cause of mortality in growing turkeys 6-12 weeks of age. In Ontario, this condition is seen at all times of the year. In this case, E. coli was recovered in large numbers from blood filtering organs. In addition, viral inclusions determined to be adenovirus (hemorrhagic enteritis virus/HEV) on TEM were identified in the splenic tissue from some of these birds. No overt intestinal hemorrhage was seen at necropsy. Larsen, et al, reported that mortality due to colibacillosis is exacerbated by earlier infection with hemorrhagic enteritis virus (Larsen et al: Colibacillosis of turkeys exacerbated by hemorrhagic enteritis virus. Laboratory Studies. Avian Diseases: 1985, vol 29: 729-732). Infection with HEV results in immunosuppression causing increased susceptibility to secondary bacterial infections such as E. coli.
AFIP Diagnosis: Spleen: Lymphoid depletion and necrosis, diffuse, severe, with reticuloendothelial cell hyperplasia and eosinophilic to amphophilic intranuclear inclusion bodies, turkey, avian, etiology consistent with adenovirus.
Conference Note: Hemorrhagic enteritis (HE) of turkeys, marble spleen disease (MSD) of pheasants, and avian adenovirus group II splenomegaly (AAS) of chickens are caused by type II adenoviruses. Turkeys, chickens, and pheasants are the only known natural hosts for members of the HE-MSD-AAS group of adenoviruses. Experimental infections have demonstrated that HE virus will produce splenic lesions in chickens and pheasants; likewise, MSD and AAS virus isolates will infect turkeys.
Hemorrhagic enteritis virus is transmitted by the fecal-oral route and has been recovered from contaminated bedding. Transmission through the egg is not believed to occur. Generally, poults 4 weeks or older are affected. Gross lesions consist of pale tissues due to anemia, blood-filled intestines, congestion of the intestinal mucosa and lungs, and splenomegaly. Histologically, there is severe congestion of the intestinal mucosa, with necrosis and sloughing of epithelial cells at the tips of villi. The lamina propria is infiltrated by macrophages, plasma cells, and heterophils. Lesions are often more pronounced in the duodenum; however, less severe lesions can occur in the proventriculus, gizzard, large intestine, cecum, cecal tonsils, and bursa of Fabricius. Splenic lesions consist of hyperplasia of white pulp and reticuloendothelial cells, as well as necrosis of lymphoid cells. Reticuloendothelial cells often contain intranuclear viral inclusions.
Infection with HE virus causes a transient immunosuppression. Colibacillosis, paramyxovirus infection, and chlamydiosis have all been reported as significant sequelae to HE infection.
Contributor: Veterinary Lab. Services, Box 3612, Guelph, Ontario, Canada N1H-6R8.
References:
1. Cheville N and S. Sato. Pathology of adenoviral infection in
turkeys (Meleagris gallopava) with respiratory disease and colisepticemia.
Vet. Pathol. 14:567-581, (1977).
2. Cheville, N. Ultrastructural Pathology: An Introduction to Interpretation. Chapter 11. Cytopathology of Viral Diseases, Pg. 514-519, (1994).
3. Larsen CT, CH Domermuth, DP Spnenberg, WB Gross. Colibacillosis of turkeys exacerbated by hemorrhagic enteritis virus. Laboratory Studies. Avian Diseases. 29:729-732, 1985.
4. Gross, WB and CH Domermuth. Spleen lesions of hemorrhagic enteritis of turkeys. Avian Diseases. 20:455-466 1976.
5. Spnenberg DP, Domenuth, CT Larsen. Field outbreaks of colibacillosis of turkeys associated with hemorrhagic enteritis virus. Avian Diseases. 29:729-732, 1985.
6. Domermuth CH and Gross WB: Hemorrhagic enteritis and related infections in Diseases of Poultry. Iowa State Univ. Press, Ames, Iowa, 9th edition, pp. 567-571, 1991.
International Veterinary Pathology Slide Bank: Laser disc frame #5334, 9244, 14531, 20508-10, and 21229.
Signalment: 10-year-old Percheron gelding.
History: Acute colic due to large colon displacement. Displacement was surgically corrected but horse could not stand after surgery. The horse was euthanized 24 hours later.
Gross Pathology: The large muscles of both hind limbs were slightly pale.
Laboratory Results: None
Contributor's Diagnosis and Comments: Diagnosis: Skeletal muscle: Severe chronic degenerative myopathy with glycogen and polysaccharide storage (equine polysaccharide storage myopathy).
Slides submitted are either #5 (biceps femoris) or #9 (rectus femoris). On both slides, there is a transverse and a longitudinal section of skeletal muscle. On a few slides, the longitudinal section consists of only a few fibers with minimal lesion. On all transverse sections, and on available longitudinal sections, there is moderate to severe fiber size variation with increased numbers of internal nuclei. Myofibers are separated by a clear zone (edema, presumptive; possible autolysis). Numerous fibers contain pale to dark, amphophilic to basophilic, subsarcolemmal or internal loculated, aggregates of material that often replaces almost the entire diameter of the myofiber. Occasional fibers contain multiple clear cytoplasmic vacuoles. There is prominent segmental necrosis with macrophage infiltration of affected segments. Regenerating fiber segments are rare. There is mild (slide #9) to moderate (slide #5) patchy endomysial fibrosis and mild fatty infiltration (slide #5).
Periodic acid-Schiff (PAS) staining for glycogen reveals large granular aggregates of glycogen within many fibers and strong positive staining of the more organized aggregates visible on hematoxylin and eosin (H&E) stained sections. Following amylase digestion, the granular aggregates are largely amylase-sensitive, whereas the more organized aggregates are generally amylase-insensitive. Many macrophages contain PAS-positive material which is partially amylase-sensitive.
This case of equine polysaccharide storage myopathy affecting Percheron and Belgian horses was identified retrospectively. At the time of this writing, this disease has been identified at this institution in four Percherons and four Belgians of both sexes. Age ranges from 2 years to 21 years. Two affected horses (aged 2 and 3 years) presented with obvious weakness, muscle atrophy, and increased recumbency. The other six horses had no clinical signs prior to acute onset of recumbency and inability to rise.
A similar disease has been reported in a draft horse by Hulland (1993) affecting a Percheron mare (personal communication). Valberg, et al., describe an identical histologic lesion in Quarter horse type breeds with a history of recurrent rhabdomyolysis (Valberg et al., 1992). This investigator has also identified this disease in a Quarter horse type that became "stiff" after a regular exercise, and the following morning was found recumbent, unable to rise.
Exertional myopathy and/or exercise intolerance is a feature of human glycogen and polysaccharide storage myopathies due to defects in enzymes involved in carbohydrate metabolism (Cornelio and DeDonatos, 1985). An enzyme defect is suspected in both the draft breeds and the quarter horse type breeds, but to date a specific enzyme defect has not been identified (Valberg et al., 1992; Valentine, unpublished observation). An autosomal recessive mode of inheritance is suspected in affected Quarter horse type breeds (Valbert et al., 1995).
The case presented is a florid case with obvious storage material present on H&E stained sections. In other cases, this polysaccharide storage material is less obvious, and requires careful searching and PAS staining for diagnosis. In all cases, there are chronic myopathic changes, including excessive fibers size variation, type 2 fiber hypertrophy, presence of internal nuclei, and frequent separation of fibers by clear spaces thought to be edema. Muscles of the proximal hind limb are most severely and consistently affected. Amylase-insensitive polysaccharide material appears to increase with age.
AFIP Diagnosis: Skeletal muscle: Myofiber degeneration and necrosis, multifocal, moderate, with intramyocytic and intrahistiocytic granular amphophilic material, Percheron, equine.
Conference Note: Lysosomes are membrane-bound cytoplasmic organelles that are formed in the Golgi apparatus. Lysosomes sequester hydrolytic enzymes that can degrade both cellular constituents and extracellular material that has been acquired by phagocytosis or pinocytosis. Autophagosomes compartmentalize host-cell derived glycoproteins, polysaccharides, and lipids. When a phagosome fuses with a lysosome, the organic material is digested in step-wise fashion within this secondary lysosome (autophagolysosome). Indigestible debris remains as a residual body. If an enzyme required for degradation of a compound is missing from the lysosome, the partially digested compound will accumulate within the secondary phagosome. Most lysosomal storage diseases are transmitted as autosomal recessive traits.
Glycogen storage diseases result from genetically inherited deficiencies in enzymes required for glycogen synthesis or catabolism. There are many enzymes required for the catabolism of glycogen; however, only one of these, à-glucosidase, is a lysosomal enzyme. In humans, 12 types of glycogen storage diseases have been identified based on the enzyme that is deficient. Although a slightly different clinical picture is present for each type of glycogen storage disease, they can be placed into three categories depending upon the organ system(s) most affected (hepatic, myopathic, or systemic).
In veterinary medicine six forms of glycogenosis have been identified. Type I glycogenosis (Von Gieke disease) is a deficiency in glucose-6-phosphatase that results in intracytoplasmic accumulations of glycogen and some lipid; it has been described in dogs. Type II glycogenosis (Pompe disease) is caused by a lack of à-glucosidase (acid maltase), and has been reported in Brahma and Shorthorn cattle, Corriedale sheep, Domestic Shorthair cats, and Japanese quail. Type III glycogenosis (Cori disease) is the result of a deficiency in amylo-1,6-glucosidase and has been described in German Shepherd Dogs. Type IV glycogenosis (Anderson disease) has been reported in dogs and is caused by a deficiency in branching enzyme. Lastly, type VII glycogenosis (Tauri disease) is caused by a deficiency in phosphofructokinase and has been reported in English Springer Spaniels.
Myopathic forms in humans include type V glycogenosis (McArdle syndrome), type VII glycogenosis, and the adult form of type II glycogenosis (Pompe disease). Clinically, these syndromes present with muscle cramps following exercise and a failure of exercise-induced rise in blood lactate levels due to a block in glycolysis.
Contributor: Dept. of Pathology, College of Veterinary Medicine , Cornell University, Ithaca, NY 14853-6401.
References:
1. Cornelio F, DiDonato S. Myopathies due to enzyme deficiencies.
J Neural 232:329-340, 1985.
2. Hulland TJ. Metabolic Myopathies. In: Jubb, Kennedy, Palmer eds. Pathology of Domestic Animals, vol.1 4th ed., New York: Academic Press, P. 216, 1993.
3. Valberg SJ, Cardinet GH III, Carlson GP, DeMauro S. Polysacchride storage myopathy associated with recurrent Exertional rhabdomyolysis in horses. Neuromusc Dis 2:351-359, 1992.
4. Valberg SA, Geyer C, Sorum SA, Cardinet GH III. Familial incidence of polysaccharide storage myopathy and Exertional rhabdomyolsis in quarter horse-related breeds. J Vet Int Med (Abstract) 9:224, 1995
5. Cotran RS, Kumar V, Robbins SL: Pathologic Basis of Disease. W.B. Saunders Co., 5th edition, pg. 146-147, 1994.
6. Summers BA, Cummings JF, and de Lahunta A: Veterinary Neuropathology. Mosby, St. Louis, pp. 214-236, 1995.
* The American Veterinary Medical Association and the American College of Veterinary Pathologists are co-sponsors of the Registry of Veterinary Pathology. The C.L. Davis Foundation also provides substantial support for the Registry.