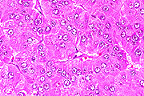
 Hepatocellular carcinoma in
a woodchuck infected with Woodchuck Hepatitis virus. Note loss
of plate architecture. (400X, HE, 145K)
Hepatocellular carcinoma in
a woodchuck infected with Woodchuck Hepatitis virus. Note loss
of plate architecture. (400X, HE, 145K)Signalment: Adult woodchuck.
History: This case was retrieved from the archival material of the Registry of Veterinary Pathology.
Gross Pathology: none submitted.
Laboratory Results: none submitted.
Contributor's Diagnosis and Comments: NA
AFIP Diagnosis: 1. Liver: Hepatocellular carcinoma, woodchuck (Marmota monax), rodent.
2. Liver: Hepatitis, portal and periportal, bridging, lymphoplasmacytic, diffuse, moderate, with piecemeal necrosis, biliary hyperplasia, cholestasis, and vacuolar change.
Conference Note: Woodchuck hepatitis virus (WHV) is a DNA hepadnavirus that causes hepatitis in woodchucks and is strongly associated with the development of hepatocellular carcinoma in infected animals. Hepadnaviruses also cause hepatitis in man (human hepatitis B virus), ground squirrels, and the Beijing duck. Like WHV, human hepatitis B virus (HBV) is also associated with an increased incidence of hepatocellular carcinoma in man. Of woodchucks trapped in Pennsylvania, Maryland, and New Jersey, 16% had titers for WHV antigens and 30% were positive for antibody to woodchuck hepatitis surface antigens, suggesting that WHV is a prevalent infection in the wild.
In man, infection with HBV can produce a spectrum of hepatic disease: acute hepatitis, chronic nonprogressive hepatitis, progressive chronic hepatitis resulting in cirrhosis, fulminant hepatitis with massive necrosis, and an asymptomatic carrier state with or without progressive disease. Woodchucks also develop acute and/or chronic hepatitis after infection with WHV; however, progression to cirrhosis is rare. Histologically, acute hepatitis due to WHV is characterized by an inflammatory infiltrate within the limiting plate of hepatocytes, with little or no hepatocellular necrosis and minimal fibroplasia and biliary hyperplasia. As the hepatitis becomes chronic, abundant inflammatory cells expand portal areas and extend beyond the limiting plate into the parenchyma. There is proliferation of bile ducts, fibroplasia, and hepatocellular necrosis, including piecemeal necrosis. It is believed that the majority of hepatocellular damage is induced by immunologic mechanisms, particularly through the action of cytotoxic T lymphocytes.
Nearly 100% of woodchucks experimentally infected with WHV develop hepatocellular carcinoma. Development of hepatocellular carcinoma is believed to be a multifactorial process involving protooncogenes, growth factor, and tumor suppressor genes. Alterations in myc oncogenes and p53 suppressor genes are implicated in WHV associated hepatocellular carcinoma; however, these alterations have not been found in human hepatocellular carcinoma. Also involved in the viral induction of neoplasia is the regulatory protein, HBx. HBx is a product of HBV's X gene and has been shown to disrupt normal growth control of infected hepatocytes by transcriptional activation of host proto-oncogenes. HBx also mimics the action of the tumor promoter TPA by activating protein kinase C, a component of signal transduction pathways. Additionally, altered autocrine regulation of cell growth possibly involving insulin-like growth factor may contribute to the development of hepatocellular carcinoma. Paracrine mechanisms for the control of growth may also be changed as a result of the synthesis and secretion of interleukins, fibroblast growth factors, and hepatocyte growth factors in response to the inflammatory and fibroplastic changes induced by viral infection.
Development of similar hepatic lesions in humans and woodchucks with hepadnaviral hepatitis, along with an increased incidence of hepatocellular carcinoma, makes the woodchuck a valuable animal model for the study of the pathogenesis of viral hepatitis and viral hepatocarcinogenicity.
References:
1. Cotran RS, Kumar V, and Robbins SL: Pathologic basis of disease.
W.B. Saunders, Co., Philadelphia, pp. 289 and 844-846, 1994.
2. Rogler CE and Chisari FV: Cellular and molecular mechanisms of hepatocarcinogenesis. Seminars in liver disease, 12:3, pp. 265-276, 1992.
3. Snyder RL, and Summers J: Chronic hepatitis and hepatocellular carcinoma associated with Woodchuck hepatitis virus. Am Jour Pathol, 107:3, pp. 422-425, 1982.
4. Popper H, Roth L, Purcell RH, Tennant BC and Gerin JL: Hepatocarcinogenicity of the woodchuck hepatitis virus. Proc Natl Acad Sci, 84, pp. 866-870, 1987.
5. Tyler GV, Snyder RL, and Summers J: Experimental infection of the woodchuck (Marmota monax monax) with Woodchuck hepatitis virus. Lab Invest, 55:1, pp. 51-55, 1986.
6. Frommel D, Crevat D, Vitvitsky L, Pichoud C, Hantz O, Chevalier M, Grimaud J-A, Lindberg J, and Trepo CG: Immunopathologic aspects of woodchuck hepatitis. Am Jour Pathol, 115:1, pp. 125-134, 1984.
7. Peters DN, Steinberg H, Anderson WI, Hornbuckle WE, Cote PJ, Gerin JL, Lewis RM, and Tennant BC: Immunopathology of glomerulonephritis associated with chronic woodchuck hepatitis virus infection in woodchucks (Marmota monax). Am Jour Pathol, 141:1, pp 143-152, 1992.
International Veterinary Pathology Slide Bank: Laser disc frame #13036.
Signalment: Adult male hairless guinea pig.
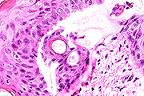 Microblister formation in a
guinea pig exposed to sulfur mustard vapor (HE, 400X, 102K)
Microblister formation in a
guinea pig exposed to sulfur mustard vapor (HE, 400X, 102K)
History: Animals were exposed to threshold microblistering doses of HD vapor (sulfur mustard) on multiple sites on their backs. Vapor caps were left in place for 5-7 minutes. Animals were sacrificed 24 hours post exposure and biopsies of exposure sites were taken.
Gross Pathology: Exposed areas exhibited erythema and edema just prior to euthanasia.
Laboratory Results: None submitted.
Contributor's Diagnosis and Comments:
Morphologic Diagnosis: Dermatitis, acute, focal to multifocal to diffuse, minimal to severe, with varying degrees of epidermal necrosis (basal cell layer, primarily) and microblister formation (epidermal/dermal separation) (note: There is some variation of the lesion among the slides submitted).
Much work has been done at this institute to determine the sequence of events leading to the development of the sulfur mustard (HD) lesion. Direct contact with sulfur mustard produces coagulation necrosis of the epidermis which extends into the superficial dermis. A vapor exposure model was developed to better study the gradual formation of the microblister. By varying the amount of time the vapor cap remains in place, one can determine the threshold dose at which the microblister occurs.
Prior to microblister formation, the cells of the lower strata of the epidermis (primarily the basal cells) become extremely swollen and occasionally rupture, beginning with a 3-minute exposure (Braue et al.) As the cells rupture, adjacent spaces coalesce to form small clefts and microvesicles. Microvesicles are initially observed in a few biopsy specimens at a 3-minute exposure and occur in 100% of the biopsy specimens at an exposure of 9 minutes. Epidermal neutrophils appear in a few sites following a 4-minute exposure and are seen in all sites following a 9-minute exposure, almost paralleling the dose-response curve of basal cell necrosis. As the time of exposure increases, so does the incidence of dermal changes, which include edema, vascular congestion, vascular necrosis, and hemorrhage.
Ultrastructural studies have shown that the first morphologic changes occur 6-8 hours following exposure to HD and that changes in the basal lamina and the anchoring filaments of the hemidesmosomes contribute to microblister formation (Petrali et al.).
Electron microscopy (2x2 Slides):
#1 Scanning EM of sulfur mustard-induced microvesicle: Microvesicle with infiltrating inflammatory cells separating epidermis from the dermis at the dermal-epidermal junction.
#2 Transmission EM of sulfur mustard-induced microvesicle: Disabled hemidesmosomes at the dermal-epidermal junction. Degenerating basal cells at the roof of the vesicle; lamina densa of the basement membrane at the floor.
AFIP Diagnosis: Haired skin: Degeneration and necrosis, epidermal, diffuse, with intraepithelial pustules, microblisters (dermo-epidermal separation), moderate acute dermatitis, and dermal edema, hairless guinea pig, rodent.
Conference Note: Sulfur mustard gas (HD) is a chemical warfare agent that was widely utilized in World War I and was recently directed against Iranian soldiers and Kurdish civilians in Iraq. Human dermal exposure to HD results in the formation of fluid-filled blisters which are incapacitating, persistent and slow to heal. Development of an antivesicant to protect human skin from HD has been hindered by the lack of a suitable animal model. The hairless guinea pig is being evaluated to determine its cutaneous response to HD exposure.
As the contributor notes, the basal epithelial cells are affected first and most severely. Ultrastructural changes include nuclear condensations, blebbing of the perinuclear envelope, defects of the plasmalemma, loss of cellular organelles, lipid inclusions, increased lysosomal activity, and necrosis. In addition, there are detachments of hemidesmosomes from the basement membrane and edema of the lamina lucida.
Recent research has implicated bullous pemphigoid antigen (BPA) as a possible site of HD activity. Bullous pemphigoid antigens 1 and 2 are noncollagenous proteins within the keratinocyte that have been identified as components of hemidesmosomes. Autoantibodies directed against BPA result in the subepidermal blister characteristic for bullous pemphigoid. Hairless guinea pigs exposed to HD lost or had diminished immunoreactivity to BPA at areas of vesication. The loss of BPA may be responsible for the loss of epithelial adhesion to the basement membrane and resultant blister formation.
These skin sections also demonstrate follicular ectasia, atrichia, and follicular orthokeratosis. These findings are considered normal for hairless guinea pigs; similar changes are also found in nude mice.
Contributor: U.S. Army Medical Research Institute of Chemical Defense, Aberdeen Proving Ground, MD 21010-5425.
References:
1. Petrali JP, Oglesby SB, and Hamilton TA: Mustard-gas skin lesion and bullous pemphigoid antigen. 52nd annual meeting of the microscopy society of America, San Francisco Press, Inc., pp. 254-255, 1994.
2. Petrali JP, Oglesby SB, Mills KR: Ultrastructural correlates of sulfur mustard toxicity. Jour Toxicol, 9:3, pp. 193-214, 1990.
3. Braue EH, Koplovitz I, Mitcheltree LW, Clayson ET, Litchfield MR, and Bangledorf CR: Charactization of the sulfur mustard vapor induced cutaneous lesions on hairless guinea pigs. Toxicology Methods, 2:4, PP. 242-254, 1992.
4. Cheville NF: Normal cell structure: An overview in Ultrastructural pathology. Iowa State University, Ames, pp. 44, 1994.
Signalment: 9-year-old male Bichon Frise.
History: The dog was presented to the referring veterinarian because of a slowly progressive, 3-month-long reluctance to walk which had been partially responsive to cage rest and treatment with corticosteroids. Physical examination of the dog revealed paraparesis with increased muscle tone in the hind limbs, exaggerated hind limb reflexes, and postural deficits. Radiographs of the dorsal spinous processes of the sixth and seventh thoracic vertebrae were characterized by a motheaten radiolucency. An extradural mass at the same level was indicated by a myelogram. A dorsal laminectomy was performed, and the mass was debulked. Specimens submitted for histological evaluation were obtained from the dorsal spinous processes and extradural mass.
Gross Pathology: The surgical biopsy specimens consisted of three irregularly shaped fragments of brown granular tissue, the largest of which was 1.4 cm long, and two pieces of bone, the larger of which was 3.0 cm in maximum dimension.
Contributor's Diagnosis and Comments:
Microscopic Findings: Dorsal spinous process of sixth thoracic vertebra: Its architecture is effaced by an infiltrative intramedullary mass that is composed of indistinct, variably-sized, occasionally centrally necrotic lobules of tightly packed monotypic columnar epithelial cells. The neoplastic epithelial cells are characterized by a plump oblong euchromatic to coarsely stippled heterochromatic nucleus, prominent nucleolus, tapered eosinophilic finely granular cytoplasm, occasional empty, sharply defined, nucleus-displacing cytoplasmic vacuoles, and indistinct cell boundaries. Some of the lobules are delimited by a prominent palisade-like arrangement of the neoplastic cells. The epithelial cell formations are separated by variably-sized moderately cellular collagenous septa. Resorption of pre-existent cortical and trabecular lamellar bone and "reactive" periosteal and cortical/trabecular endosteal neo-osteogenesis are also apparent.
T6-T7 extradural mass (not submitted): The specimen consists of three irregularly shaped fragments of fibrofatty tissue, within which there is an infiltrative multinodular mass that is similar in appearance to the abnormality observed in the dorsal spinous process.
MORPHOLOGIC DIAGNOSIS: Dorsal spinous process of sixth thoracic vertebra: Metastatic Sertoli cell tumor.
After receiving a copy of the biopsy report, the submitting veterinarian called to ask if the Sertoli cell tumor could in any way be related to the seminoma that had been identified in the dog's right intra-abdominal testis which had been resected 19 months previously. He also stated that the left scrotal testis was resected at the same time but not submitted for histological examination. This "revelation" was of particular interest because the request for service form that was sent with the biopsy indicated that the dog was intact male. Furthermore, no past medical/surgical history had been provided.
Re-examination of the microscopic slides prepared from the resected cryptorchid testis confirmed the presence of a seminoma. In addition, a solitary 250x microscopic field was characterized by a nodular array of vacuolated columnar cells. This abnormality was consistent with the appearance of a Sertoli cell tumor and was identical to the metastatic foci observed in the dog's dorsal spinous processes and spinal canal (extradural mass). The dog did poorly after surgery and was euthanized 3 months later. A post-mortem examination was not performed.
This case is illustrative of one of the challenges (namely, an incorrectly or incompletely filled out request form) commonly encountered by diagnostic pathologists involved in an outpatient biopsy service. In addition, several truisms about canine testicular neoplasia were reinforced. Primary testicular tumors are quite common in older dogs. Of the three primary testicular neoplasms in dogs, the different types occur with approximately equal frequency. Combinations of the common types of testicular tumors occur in approximately 25% of cases of canine testicular neoplasia. Canine testicular tumors are found more frequently in the right than in the left testis. This is also true for the cryptorchid testis. Cryptorchid dogs are at least ten times more likely to develop primary testicular neoplasms than are normal dogs.
Sertoli cell tumors and seminomas, although potentially malignant, rarely metastasize. Spread of this dog's intra-abdominal tumor to its thoracic spine might have been facilitated by the vertebral vein system, an alternate route by which blood from the body is returned to the heart via anastomosis with rostral systemic veins and the azygous vein. Since no valves exist in the vertebral venous plexuses, blood may flow cranially or caudally depending on pressure relations. Over 50 years ago the renowned American otolaryngologist Oscar V. Batson (1894-1979) discussed the function of the vertebral veins and their role in the spread of metastases.
AFIP Diagnosis: Thoracic vertebra, dorsal process (per contributor): Sertoli cell tumor, Bichon Frise, canine.
Conference Note: Skeletal metastasis often results in osteolysis. Tumor cells have limited osteolytic capacity, and osteoclasts, the major bone resorbing cell in the body, are not always present at the interface between the resorbing bone and the tumor. Recently, it has been shown that tumor-infiltrating macrophages (TIMs) form a major component of the inflammatory reaction associated with skeletal metastases. In vitro studies have demonstrated that the uninucleate and multinucleate TIMs are capable of lacunar bone resorption and of differentiation into osteoclast-like cells. This differentiation requires the presence of 1,25-dihydroxy vitamin D3 and osteoblast/stromal cells. It is hypothesized that TIMs may be responsible for osteolytic lesions associated with skeletal metastases.
Malignant cells may secrete factors that promote osteoclast differentiation; for example, human breast cancer cells secrete eicosanoids some of which are potent osteoclastogenic agonists. Many carcinoma cells produce macrophage colony stimulating factor, a protein required for osteoblast/stromal cell mediated osteoclastogenesis.
Regardless of the origin of the osteoclast, once activated they degrade first the mineral and then organic components of bone. The osteoclasts attach to the extracellular bone matrix via a transmembrane protein, integrin àv 3. Once attached, they degrade the inorganic component of bone by secretion of HCl. The secretion of HCl is accomplished by an H+-ATPase pump coupled with a Cl- channel. It is believed that the organic portion of bone is degraded by acidic collagenases, such as cathepsin D and L, released from the osteoclast by a lysosomal delivery system.
Contributor: Angell Memorial Animal Hospital, 350 S. Huntington Ave., Boston, MA 02130.
References:
1. Evans HE: 1993, Veins. In: Miller's anatomy of the dog, ed.
Evans HE, 3rd ed., pp682-716. WB Saunders, Philadelphia, PA.
2. Ladds PW: 1993, The male genital system. In: Pathology of domestic animals, Vol 3, eds. Jubb KVF, Kennedy PC, Palmer N, 4th ed., pp471-529. Academic Press, San Diego, CA.
3.Teitelbaum SL: Editorial: Mechanisms of tumor-induced osteolysis. Laboratory Investigation, 71:4, pp. 453-455, 1994.
4. Quinn JMW, Matsumura Y, Tarin D, McGee JO'D, and Athanasou NA: Cellular and hormonal mechanisms associated with malignant bone resorption. Laboratory Investigation, 71:4, pp. 465-471, 1994.
5. Okada H, Merryman JI, Capen CC, and Rosol TJ: Ultrastructural and Histomorphometric evaluations of gallium nitrate on bone in nude mice bearing a canine adenocarcinoma (CAC-8) model of humoral hypercalcemia of malignancy. Vet Pathol 32:36-42, pp. 36-42, 1995.
International Veterinary Pathology Slide Bank: Laser disc frame #898, 903, 2264, 4708, 4787-8, 5721, 6076, 6374, 6593, 9150, 9274, 9620, 11908, 14750, 16614-20, 16624, 16647-9, 16667, 21429, and 22239.
Signalment: 60-day-old male castrated Yorkshire x Landrace pig.
History: This tissue is from a pig fed an excess level of a required nutrient for 27 days as part of a research trial.
Gross Pathology: The pig was severely lame but in good condition. The skin was dry and scurfy, oral mucocutaneous junction was severely reddened and pinna had focal severe ulceration and exudation at site of ear tag. Many long bones had multifocal, usually centrally located closure of growth plates which were sometimes, but not always moderately narrowed. Growth plates of the rib and distal ulna were severely to moderately thickened. The axial skeleton was not affected.
Laboratory Results: Post-trial, a CBC and complete chemistry panel were done on all pigs. The only significant differences found were elevation in total serum protein and decrease in packed cell volume of pigs fed the excess nutrient.
Contributor's Diagnosis and Comments:
Morphologic Diagnosis and Etiology: 1. Premature metaphyseal growth plate closure, humeral head, multifocal, moderate, acute to subacute, with necrosis. 2. Ossification of resting and proliferation zones of epiphyseal growth cartilage of the articular-epiphyseal cartilage complex, humeral head, perivascular multifocal, mild. 3. Hypertrophy and hyperplasia of hepatic Ito cells, diffuse, severe.
Etiology: Vitamin A Toxicosis.
There are many causes of premature closure of growth plates. In field cases it is often difficult to ascertain the etiology. A study of vitamin A toxicosis was undertaken to better describe, identify and hopefully understand the pathogenesis of one such agent.
The tissue submitted was from one of five pigs fed a wheat-based starter ration containing 875,000 IU of vitamin A (acetate)/kg of feed (500 x NRC). The combination of premature closure of growth plates plus hepatic Ito cell hypertrophy and hyperplasia may be of some benefit in helping diagnose field cases. The significance of the small bony spicules in the resting or proliferating zone of the epiphyseal cartilage around epiphyseal vessels of the articular-epiphyseal cartilage complex is unknown.
AFIP Diagnosis:
1. Humerus (per contributor): Closure of physeal growth plate,
multifocal, Landrace-Yorkshire cross, porcine.
2. Liver, Ito cells: Hyperplasia, diffuse, moderate.
Conference Note: Vitamin A toxicity is characterized by premature closure of physeal growth plates, osteoporosis, and development of exostoses. Baby pigs and kittens fed excess vitamin A demonstrate shortening of long bones, prolongation of the traction epiphyses, and rotation of epiphyses. Histologically there is a decrease in the width and depth of the physeal growth plates due to reduced chondrocyte proliferation and reduced size of hypertrophic chondrocytes. In severe cases there is often premature closure of the growth plate; the fastest growing physes are most affected.
Vitamin A toxicity also induces osteoporosis characterized by a decrease in numbers of osteoblasts and fewer, thinner osteoid seams. The effect is more severe in periosteal formation of bone, leading to thin cortices with an emphasized metaphyseal flare. The formation of exostoses is characteristic of vitamin A excess in adult animals; this is best illustrated by deforming cervical spondylosis in cats.
This slide was compared to a section from the proximal humerus of an age/nutrient matched control pig from the same experiment. There are no prominent eosinophilic streaks about the physeal vessels of the control; these are striking in the experimental case. The proliferating zone of the control physis is slightly wider, and the hypertrophied chondrocyte columns are longer and thinner, with substantially less matrix production. The cartilage cores of the primary spongiosum in the hypervitaminosis A physis are thicker and more anastomosing when compared to the control physis. The is a greater concentration of osteoclasts/chondroclasts in the primary spongiosum of the control physis; however, the cortical cutback zones of both physes are similar.
The pathogenesis of vitamin A toxicity as it relates to endochondral and periosteal bone formation is not completely understood. Vitamin A has been shown to inhibit chondrocyte proliferation and reduce RNA and protein synthesis. Recent in vitro experiments have determined that retinoic acid, a derivative of vitamin A, is necessary for maturation of physeal cartilage. When retinoic acid was supplied to collagen producing chondrocytes, they increased production of alkaline phosphatase, osteonectin, and osteopontin. Osteonectin and alkaline phosphatase are glycoproteins necessary for mineralization of cartilage. Osteopontin is a sialoprotein found in many bone cells; its function is unknown. These results suggest that an abnormal chronology of bone matrix formation may be responsible for the skeletal lesions observed in vitamin A intoxication.
Vitamin A is hydrolyzed in the gut to retinol, bound to lipid, and stored in Ito cells in the liver. The number of Ito cells found in the livers of control pigs and pigs fed excess vitamin A correlated with the tissue levels of vitamin A present.
There is also a multifocal mild histiocytic and lymphoplasmacytic hepatitis and multifocal minimal extramedullary hematopoiesis in the liver sections from this case. These findings were considered insignificant and probably normal for a 2-month-old pig.
Contributor: University of Saskatchewan, Saskatoon, Saskatchewan S7N 0W0 Canada.
References:
1. Biesalski, H.K.: Comparative assessment of the toxicology of Vitamin A and retinoids in man. Toxicology 57: 117-161, 1989.
2. Jones, E.A.: Hepatic sinusoidal cells: new insights and controversies. hepatology 3: 259-266, 1983.
3. Wokle, R.E.; Neilsen, S.W.; Rousseau, J.E.: Bone lesions of hypervitaminosis A in the pig. Am. J. Vet. Res. 29: 1009-1024, 1968.
4. Iwamoto M, Yagami K, Shapiro IM, Leboy PS, etal: Retinoic acid is a major regulator of chondrocyte maturation and matrix mineralization. Microsc Res Tech, 15:28(6), pp. 483-491, 1994.
Dana P. Scott
Captain, VC, USA
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: Scott@email.afip.osd.mil
* The American Veterinary Medical Association and the American College of Veterinary Pathologists are co-sponsors of the Registry of Veterinary Pathology. The C.L. Davis Foundation also provides substantial support for the Registry.