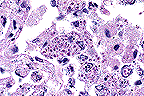
 Numerous intracellular yeasts
(H. capsulatum) within alveolar macrophages in a llama.
(40X, PAS, 83K)
Numerous intracellular yeasts
(H. capsulatum) within alveolar macrophages in a llama.
(40X, PAS, 83K)
Signalment: 4-year-old pregnant female llama
History: This llama was one of several imported from Bolivia to Indiana. The llamas were held in quarantine for two months, five months after which this animal became emaciated and depressed. The animal had a neutrophilia with a left shift and was treated with intravenous fluids, penicillin, and gentamicin. Cytologic examination of a tracheal aspirate and bronchoalveolar lavage fluid revealed neutrophils and macrophages with intracytoplasmic, 2-4 æm, round structures. The llama died three days later.
Gross Pathology: The llama had a thin body with marked subcutaneous ventral edema in the abdominal area. The pleural cavity contained one liter of clear yellow fluid. The lung had numerous, multifocal, 3-6 mm diameter, firm, white foci that were sometimes cystic, fluid-filled with free floating friable masses. Similar white foci were also present in the liver and in skeletal muscle throughout the body. The uterus contained a small amount of purulent exudate.
Laboratory Results: Tissues were sent to the National Veterinary Services Laboratory, Ames, Iowa, where histopathologic, ultrastructural, and immunofluorescent (Dr. Francis Chandler, Medical College of Georgia; Dr. Leo Kaufman, Center for Disease Control, Atlanta, Georgia), characteristics were determined to be most representative of Histoplasma capsulatum. Culture of the organism was unsuccessful; Candida glabrata was isolated but regarded as a contaminant.
Contributor's Diagnosis and Comments: Pneumonia, multifocal, necrotizing, and granulomatous, severe with intralesional yeasts compatible with Histoplasma capsulatum.
Histoplasma capsulatum was the apparent cause of the disseminated pulmonary lesions in this llama. This may be the first case of this agent in llamas. Infection of human beings with histoplasmosis occurs in South America, but it has not been reported in Bolivia where this llama originated. It is thought that the fungus cannot survive in the cool, arid climate of that country. Indiana, in contrast, is an area endemic to histoplasmosis. The stress of importation, pregnancy, and other possible immunosuppressive factors may have led to increased susceptibility and fatal pulmonary infection.
Histologically, sections of lungs contained numerous oval to irregularly shaped foci of caseous necrosis surrounded by marked infiltrates of macrophages and moderate numbers of neutrophils. Macrophages contained large numbers of 2 to 4 æm, round to oval, intracytoplasmic structures containing a single, usually eccentric, nucleus. Airways adjacent to the lesions contained moderate numbers of neutrophils and macrophages, many of which contained similar organisms. These organisms stained black with Gomori's methenamine silver stain, pink with periodic acid-Schiff (PAS) and did not stain with Giemsa stain. Narrow based buds were seen in special stains. No identifiable organisms were present in sections of liver.
Ultrastructurally, intracellular and extracellular organisms (1.0-1.9 x 1.6-4.0 æm) had small eccentric nuclei with mitochondria, vacuoles, whorled membranes, and occasional lipid droplets. The organisms were surrounded by an electron translucent fibrillar wall and a dense fibrillar outermost layer. Several organisms had bud-like projections. They lacked evidence of a kinetoplast and non-emergent flagellum typical of the amastigote stage of Leishmania sp.
AFIP Diagnosis: Lung: Pneumonia, pyogranulomatous, necrotizing, multifocal, moderate, with intrahistiocytic yeast, llama (Lama glama), camelid, etiology consistent with Histoplasma capsulatum.
Conference Note: Histoplasma capsulatum is a dimorphic fungus that grows as a nonparasitic, mycelial form in the soil and a parasitic, budding yeast in animals with body temperatures between 30 and 37oC. The mycelial form produces spherical microconidia ranging in size from 2-4 m, and club shaped macroconidia which range from 8 to 14 m . In tissue, H. capsulatum is a 2-4 m spherical or oval yeast that reproduces by narrow-based budding. Histopathological processing often causes the cytoplasm of H. capsulatum to shrink from the cell wall, creating a clear halo.
Histoplasma infection is initiated when the microconidia are inhaled. Inhaled particles must be about 2 m in diameter or smaller to reach the alveoli. Larger particles are trapped and removed from the respiratory tract by the mucociliary elevator. The small size of histoplasmal microconidia allows them to by-pass the mucociliary elevator and colonize the lung. Once in the alveoli, the microconidia convert to the yeast form and are phagocytized by pulmonary macrophages. In most instances, H. capsulatum causes a self-limiting pneumonia. In susceptible animals, however, the organism is spread by macrophages within the lymphatics and then the circulatory system. These animals develop a disseminated disease which can affect many organs including the central nervous system, intestine, adrenal glands, skeletal system, heart, and kidneys. It has also been hypothesized that the mycelia or conidia of H. capsulatum can infect the intestine after being ingested.
Contributor: National Animal Disease Center, P.O. Box 70, 2300 Dayton Road, Ames, IA 50010.
References:
International Veterinary Pathology Slide Bank:
Laser disc frame #5294, 5295, 5758, 13128.
 . Pulmonary Yersinia pestis
infection in a monkey. (40X, HE, 88K)
. Pulmonary Yersinia pestis
infection in a monkey. (40X, HE, 88K)
 . Pulmonary Yersinia pestis
infection in a monkey. (100X, BH, 118K)
. Pulmonary Yersinia pestis
infection in a monkey. (100X, BH, 118K)
Signalment: Adult female African green monkey (Cercopithecus aethiops)
History: Twenty-three adult African green monkeys died after experimental exposure to either inhaled F1-positive or F1-negative strains of Yersinia pestis. This monkey was experimentally exposed to 32,000 inhaled colony-forming units of Y. pestis strain Java-9. The monkey died five days postexposure.
Gross Pathology: A severe multilobar pneumonia with serosanguineous pleural effusion and minimal fibrinous pleuritis was present. The multilobar pneumonia was characterized by multiple red, heavy, edematous lobes that failed to collapse. Within the involved lung lobes, there were spherical to discoid necrohemorrhagic foci up to 2 cm in diameter.
Laboratory Results: Yersinia pestis was recovered by bacteriological culture from cardiac blood collected at necropsy.
Contributor's Diagnosis and Comments:
Etiology: Yersinia pestis strain Java-9
Plague is an acute febrile disease caused by Yersinia pestis. There are three clinicopathologic forms of human plague: bubonic, primary septicemic, and primary pneumonic. Primary pneumonic plague in humans generally follows a 48-60 hour incubation period. Its onset is marked by sudden chills, high fever, severe cough, and dyspnea. The sputum is watery, frothy, occasionally bloody, and teeming with bacteria. Often, the patient dies within one to two days after the onset of clinical signs. Death is due to acute respiratory insufficiency and/or shock from sepsis.
Primary pneumonic plague in humans begins as a bronchopneumonia characterized by numerous bacteria and a proteinaceous effusion in the alveoli. The pneumonia spreads rapidly, becoming lobar, then multilobar, and is accompanied by pleuritis. Microscopic examination reveals hemorrhage, foci of necrosis, numerous bacilli in most of the alveoli, and relatively scant suppuration in comparison to the number of bacteria.
Previous studies have characterized experimental pneumonic plague in rhesus monkeys (Macaca mulatta), a species that is not considered to be uniformly susceptible to plague. The African green monkey (Cercopithecus aethiops), is considered highly susceptible to Yersinia pestis.
The protein capsule of Yersinia pestis, known as Fraction 1 or F1, is encoded by a 100-kb plasmid, known as pFra. The F1 antigen is a protective immunogen and an assumed but not proven virulence factor. The F1 antigen is an important component of the currently licensed plague vaccine and is used in diagnostic and serologic assays. There is only one recorded human plague case caused by an F1-deficient strain. The reports on the virulence of F1-negative strains in animal models are conflicting. These F1-negative virulence studies did not use genetically defined F1-negative mutants. Therefore, it is unknown whether the F1-negative strains were lacking the F1 structural gene, F1 operon, or the entire 100-kb pFra plasmid that contains other potential virulence factors, or whether the pFra plasmid had integrated into the chromosome. Yersinia pestis strain Java-9, isolated from rats in Indonesia, is an F1-negative strain lacking the autonomous pFra plasmid.
The ability of the F1-negative Yersinia pestis strain Java-9 to cause lethal primary pneumonic plague in this African green monkey has possible public health implications. First, there may be a need for an improved plague vaccine that is not based only on F1 antigen. Second, there may be a need for diagnostic assays that are not solely based on detection of F1 antigen or detection of antibodies to the F1 antigen.
AFIP Diagnosis: Lung: Pneumonia, fibrinohemorrhagic, acute, diffuse, severe, with intra-alveolar edema, and myriad bacilli, African green monkey (Cercopithecus aethiops), primate.
Conference Note: Natural plague in man, or urban plague, is maintained by fleas which feed on rodents infected with Yersinia pestis and then feed on humans. In the U.S., the natural reservoirs of Y. pestis are wild rodents, especially ground squirrels and prairie dogs. The disease caused by Yersinia pestis in these wild rodents is referred to as sylvatic plague. Once the bacteria enters a new mammalian host, it expresses numerous virulence factors, some of which are carried on the bacterial chromosome and others that are encoded on three different plasmids. One of the plasmid encoded virulence factors, F1, has been discussed by the contributor. Another plasmid encoded virulence factor, plasminogen activator protease, allows the bacteria to spread through host tissue by lysing fibrin deposits, breaking down extracellular matrix proteins, and inactivating C3. V antigen is another virulence factor and is believed to impart resistance to phagocytosis and also regulates the expression of other plasmid encoded virulence factors. Yersinia pestis secretes a protein called YopM that binds thrombin and interferes with platelet function and generation of an effective immune response.
The contributor noted rare pulmonary fibrin microthrombi. These microthrombi are hallmarks of disseminated intravascular coagulation (DIC). Infection with Yersinia pestis often produces large numbers of bacteria and therefore large quantities of endotoxin. One consequence of endotoxemia is the initiation of DIC. The pathogenesis of disseminated intravascular coagulation due to endotoxemia has not been fully elucidated; however many of the effects of endotoxin on the vascular system are known. Macrophages, lymphocytes, and the complement system are activated by endotoxin. Endotoxin localizes in or on endothelial cells and can cause vasculitis. The mechanism by which endothelial cells are damaged is not known; however, it is not dependant upon complement. Endotoxin also activates Hageman factor (factor XII) and has been shown to reduce levels of Factor VII; therefore, it may initiate coagulation via the intrinsic or extrinsic pathways. The interaction of these factors contributes to the disseminated intravascular coagulation seen in plague. The thrombi cause ischemia and tissue necrosis. Nonenzymatic coagulation factors (V and VIII) may be consumed faster than they can be replaced, resulting in consumption coagulopathy.
Contributor: USAMRIID, Pathology Division, Ft. Detrick, MD 21702-5011.
References:
International Veterinary Pathology Slide Bank:
Laser disc frame #24653, 24654.
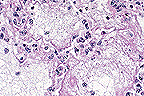 . Severe fibrinous interstitial
pneumonia due to Staph enterotoxin B inhalation. (40X, HE, 103K)
. Severe fibrinous interstitial
pneumonia due to Staph enterotoxin B inhalation. (40X, HE, 103K)
Signalment: 5.4 kg adult female rhesus monkey (Macaca mulatta).
History: This monkey died two days after receiving an inhaled dose (12 æg/kg) of staphylococcal enterotoxin B (SEB).
Gross Pathology: There was partial collapse and diffuse reddening of the lungs, frothy fluid in the airways, and mildly enlarged axillary, inguinal and mesenteric lymph nodes.
Laboratory Results: None.
Contributor's Diagnosis and Comments:
Staphylococcal enterotoxin B is one of a family of enterotoxins known as "super antigens" produced by Staphylococcus aureus. Super antigens stimulate T cells expressing distinct V subsets of antigen receptors. As a T cell mitogen, SEB binds to major histocompatibility complex (MHC) class II molecules, stimulating T cell proliferation. Primates including humans are very sensitive to the effects of SEB, perhaps because of strong MHC class II binding affinities for SEB. The systemic effects of SEB intoxication via parenteral exposure have been attributed to a massive release of cytokines such as interferon-gamma, tumor necrosis factor-alpha and interleukin-6, whereas the gastrointestinal illness following ingestion of SEB as part of staphylococcal foodborne illness is related to histamine and leukotriene release from mast cells. A distinctive morphologic finding in the lungs of monkeys with inhaled SEB intoxication is the presence of numerous large lymphocytes with mitotic figures. A recent immunolabelling study has established that many of these large lymphocytes are T cells. The pathogenesis of the massive pulmonary edema has not yet been worked out, but is hypothesized to be of the high permeability type, perhaps due to cytokine- induced increased pulmonary vascular permeability, resulting in fatal toxic shock.
AFIP Diagnosis: Lung: Pneumonia, interstitial, fibrinous, subacute, diffuse, moderate, with interstitial and alveolar edema and intravascular lymphoblasts, rhesus monkey (Macaca mulatta), nonhuman primate.
Conference Note: Superantigens bind outside of the conventional antigen groove of the MHC II molecule. This complex binds only to the -chain variable region (V ) of T cell receptors. This interaction leads to activation and proliferation of a subpopulation of cells which share the same elements of the V region. Superantigens are capable of activating 5-20 % of the body's T lymphocytes (this subpopulation shares a similar V region) while a typical antigen would activate only 0.01 per cent. The result of this over-stimulation is the release of large quantities of cytokines that result in fever, changes in vascular permeability and shock. Often there is a "burn-out" of subpopulations of affected lymphocytes, probably caused by superantigen induced apoptosis.
Pathologic conditions mediated by superantigens are not limited to staphylococcal enterotoxins. Mycoplasmal arthritis, scalded skin syndrome, toxic shock syndrome, acute rheumatoid fever, and many retroviral diseases also involve superantigens. Nor is the effect of superantigens limited to the excessive elaboration of cytokines. Superantigens induce immunodeficiency in retroviral murine acquired immunodeficiency, and a similar component may play a role in human acquired immunodeficiency. Superantigens are required for the development of reticulum cell sarcoma (B cell lymphoma) in SJL mice. Hypothetically, superantigens may be capable of inducing autoimmune diseases by activating autoreactive cytotoxic T cells, or by deletion (or inducing anergy) of a suppressor T cell subpopulation.
Contributor: USAMRIID, Pathology Division, Ft. Detrick, MD 21702-5011
References:
 . Spongiosis of the cerebellar
white matter in a polled Hereford calf. (40X, HE, 89K)
. Spongiosis of the cerebellar
white matter in a polled Hereford calf. (40X, HE, 89K)
Signalment: 2 day-old polled Hereford heifer, bovine, Bos taurus.
History: Recumbent since birth, unable to rise. Apparent blindness with normal corneal and palpebral reflexes. Generalized weakness, with head extended and neck appearing stiff.
Gross Pathology: No gross lesions.
Laboratory Results: NA
Contributor's Diagnosis and Comments:
The pattern of vacuolation affecting white matter in the brain, and grey and white matter of the spinal cord is common in Hereford calves with maple syrup urine disease. A presumptive diagnosis was based on clinical, pathologic, and genetic analysis. Biochemical analysis is available but was not pursued in this instance. Branched chain amino acids accumulate giving the urine a distinctive odor. Several other clinically similar diseases affect calves making pathologic and biochemical examination important.
AFIP Diagnosis:
Conference Note: Conference attendees noted the presence of the external germinal layer of the cerebellum and determined the animal was perinatal . Differential diagnosis included neuraxial edema, citrullinemia, bovine spongioform encephalopathy, and maple syrup urine disease.
Maple syrup urine disease (MSUD) is characterized by vacuolation of white matter tracts and, to a lesser degree, grey matter in the central nervous system. Electron microscopy has revealed intra-myelinic vacuole formation with splitting of the myelin sheath at the intraperiod lines.
Although the disease is inherited as an autosomal recessive by polled shorthorns, polled Herefords and humans, the biochemical defects differ. In polled Herefords, maple syrup urine disease results from a nonsense mutation in codon-6 of the gene for the E1à subunit of branched-chain à-keto acid dehydrogenase, a mitochondrial enzyme. Polled Shorthorns suffer a slightly different defect, also in the E1à subunit. Deficiency of branched-chain à-keto acid dehydrogenase results in elevated tissue and blood levels of the branched-chain amino acids, leucine, isoleucine, and valine and their respective keto acids, ketoisocaproic acid, keto-beta- methylvaleric acid, and ketoisovaleric acid. Calves are affected at birth, prior to suckling. The pathogenesis of the histologic lesions of MSUD and their relationship to elevated levels of branched-chain amino acids are not known.
Polled Herefords have been suggested as an animal model for MSUD in humans. However, humans suffering from MSUD are deficient in à-keto acid decarboxylase, not à-keto acid dehydrogenase, as is the case in the polled Hereford. Therefore, there are elevations of different by-products of branched-chain amino acid metabolism in human and bovine MSUD.
Contributor: North Dakota State University, Veterinary Diagnostic Laboratory, Fargo, ND 58105-5406.
References:
Dana P. Scott
Captain, VC, USA
Registry of Veterinary Pathology*
Department of Veterinary Pathology
Armed Forces Institute of Pathology
(202)782-2615; DSN: 662-2615
Internet: Scott@email.afip.osd.mil
* The American Veterinary Medical Association and the American College of Veterinary Pathologists are co-sponsors of the Registry of Veterinary Pathology. The C.L. Davis Foundation also provides substantial support for the Registry.